Light turns biomass into diesel fuel and hydrogen
Biomasses are organic residuals and waste from agriculture, forests and other human activities; they represent one of the greatest carbon sources in nature (800 Mt in dry matter biomass is harvested and used in Europe as reported by official EU sources) and they potentially integrate our renewable sources panel contributing to a decarbonated energy mix.
Despite the fact that batteries and electric vehicles are in the spotlight, nowadays liquid fuels remain a by far more effective way of storing energy, energy that can be readily used from cars to airplanes or industrial production plants.
Using biomasses to replace petrol requires efficient transformation technologies or the energetic advantage can be quickly reduced. Current thermal processes are fast but energy hungry, while biotechnological methods require time and occupy large volumes.
Photocatalytic processes exploiting light to perform the same conversion are scarcely effective: they mostly employ biomass to produce hydrogen, but the technique itself produces wastes and a part of the biomass load or another sacrificial compound is consumed to protect the catalyst itself against corrosion as reported in literature (1,2).
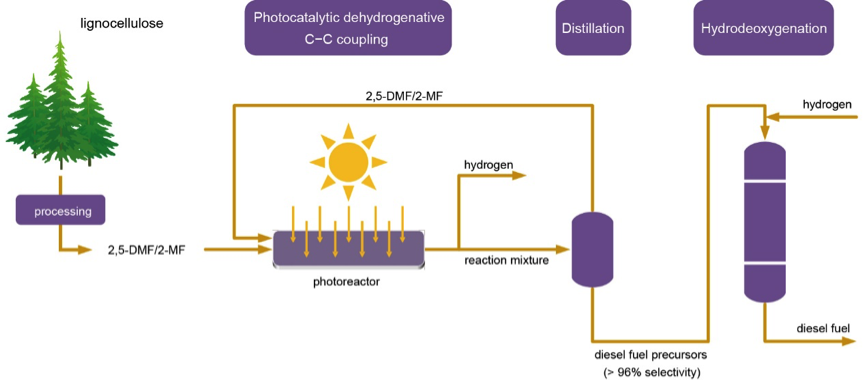
The groups of Prof. Wang of the Chinese Science Academy (China)and Prof. Fornasiero of the University of Trieste (Italy)have designed and implemented a method that turns methylfurans from lignocellulose (abundant in biomass) into diesel fuel precursor and hydrogen simultaneously. The hydrogen generated can be used to help closing the cycle and finally produce diesel fuel (see figure below).
They found that the key point of this process is the oligomerization step, which could be easily performed in a photocatalytic way using a Ru-doped ZnIn2S4catalyst. Depending on the constituent of the feedstocks, the apparent quantum yields could jump from 1.9% to a comfortable 15.2%. This is only a first but promising first step.
Understanding the structure of these catalysts before and during their operation has required time and sophisticated techniques. Among these, X-ray absorption spectroscopy has been used by Dr. Fonda at the SAMBA beamline of SOLEIL synchrotron and high resolution electron microscopy by Dr. Heggen of Forschungszentrum Juelich GmbH.
SAMBA beamline was ideally suited to study these catalytic “nanoflowers” composed of Ru (precious and used only as a dopant at 0.46mol%), Zn and In sulphide. The three elements have been studied in the suspension under illumination thanks to the sensitivity of our detectors and the penetration of hard X-rays. For instance, we have been able to correlate a partial deactivation of the catalyst during the first hours of operation with the formation of a small fraction of metallic ruthenium at the surface of the nanoflowers highlighting the importance of the Ru doping.
The capability of studying nanocatalysts, even diluted, in real operation condition is certainly one of the task we are addressing and we want to address even better in the future at synchrotron SOLEIL.
References :
1 - I. Shown, S. Samireddi, Y.-C. Chang, R. Putikam, P.-H. Chang, A. Sabbah, F.-Y. Fu, W.-F. Chen, C.-I. Wu, T.-Y. Yu, P.-W. Chung, M. C. Lin, L.-C. Chen & K.-H. Chen, "Carbon-doped SnS2 nanostructure as a high-efficiency solar fuel catalyst under visible light", Nature Communications 9, Article number: 169 (2018)
2 - V. Kumaravel, M. Danyal Imam, A. Badreldin, R.K. Chava, J. Yeon Do, M. Kang & A. Abdel-Wahab, "Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts", Catalysts 2019, 9, 276; doi:10.3390/catal9030276