Light activates a molecular virulence switch in the bacterium responsible for the “black rot” in cruciferous crops
Researchers from the Fundación Instituto Leloir in Argentina together with scientists from PROXIMA-1 & PROXIMA-2A beamlines were able to describe at a molecular level the conformational changes triggered by a light sensor of the pathogen responsible for the black rot of cruciferous plants. The study, published in the journal “Science Advances”, deciphers the general mechanisms underlying bacterial photoreception and lays the foundations for the future development of strategies that contribute to the control of this pest that causes great losses in agriculture.
For the first time, a team of Argentine scientists was able to characterize at a molecular level the different states adopted by a protein that functions as a red light sensor in the bacterium Xanthomonas campestris pv. campestris. This microorganism is responsible for the black rot, a disease that generates huge economic losses to cruciferous crops such as broccoli, Brussels sprouts, cabbage, cauliflower and radish. In previous studies the team had established that this photoreceptor regulates the virulence of the pathogen in response to changes in environmental light.
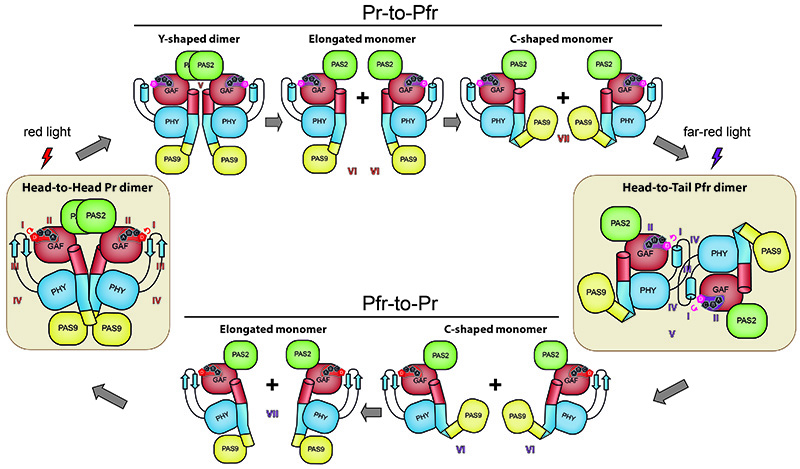
Although the team that conducted this study -led by Hernán Ruy Bonomi, Jimena Rinaldi and Lisandro Otero (Molecular Microbiology and Immunology Laboratory; Fundación Instituto Leloir)- does not work directly on antimicrobial strategies against Xanthomonas, its description of the atomic-scale changes that occur in this light sensor may serve other scientists to design interference strategies to reduce its virulence. The researchers were able to describe the structural conformation of the bacteriophytochrome “XccBphP” photoreceptor in two different states and built a model for its reversible photoconversion cycle (Figure 1). In the so-called "Pr" state, XccBphP absorbs red light as a head-to-head dimer, while in the "Pfr" state it absorbs far red light as a head-to-tail dimer. The authors also demonstrated, using biological assays, that changes in the conformation of this light receptor have effects on the virulence of X. campestris.
To describe in detail this photosensory transducing system, the scientists applied biophysical, biochemical and computational approaches like light scattering, UV-Visible spectroscopy, cross-linking, mass spectrometry, molecular dynamics simulations and X-ray crystallography, a very powerful technique to determine the structure of proteins. All the crystallographic information was collected at the PROXIMA-1 and PROXIMA-2A beamlines at SOLEIL (Figure 2).
The molecular description of the XccBphP photoreceptor helps deciphering one of the greatest enigmas about the functional mechanism of phytochromes and opens up a new horizon in the characterization of the processes that phytochromes regulate not only in bacteria but also in plants, fungi, and algae, where they are widely distributed.
The Argentine team has already performed a large number of experiments at SOLEIL for several projects since the signature of a cooperation agreement between the Argentinian Ministry of Science and the synchrotron in 2011.