Is IrO2 the best electrocatalyst to produce "green" hydrogen? Yes, but not in any state!
Iridium oxide is the key catalyst for the production of green hydrogen through water electrolysis in acid but it is very rare and expensive. In order to optimize its use as an electrocatalyst, it is of paramount importance to better understand its structure-properties relationship. By studying synthesized mixed Ir-Mo oxides with operando XAS on the SAMBA beamline, it was unambiguously evidenced that their high electrochemical activity is related to structural features: its “pseudo-amorphous" state; this structure leads to a much better activity than that of amorphous or highly crystallized materials.
The hydrogen industry is currently booming: how can we not see in this gas the energy vector of the future that can offer clean and sustainable energy? The only problem is that more than 95% of current hydrogen production still uses fossil fuels, via a process that emits about 10 kg of CO2 per kg of H2 produced. Proton Exchange Membrane (PEM) electrolyzers are among the most promising technologies for converting electricity into hydrogen in a carbon-free way.
In a PEM electrolyzer, the most complex reaction is the Oxygen Evolution Reaction (OER), which takes place at the anode at a high potential and in an acidic environment. Thus, only iridium-based electrocatalysts, and more particularly iridium oxide IrO2, are highly active and stable under these conditions. However, iridium is one of the least abundant elements on earth, hence its price is very high and doesn’t stop increasing.
Numerous studies, using operando techniques, have been published in the last 5 years aiming at understanding the efficiency of iridium oxide for OER in order to design new catalysts with lower iridium contents but still as efficient. Although it is well known that IrO2 is much more active when it is amorphous or pseudo-amorphous than when it is crystalline, these studies have not allowed to decide on the origin of this difference. Two hypotheses: the importance of the local crystallographic structure of the catalyst, or that of its electronic structure and the presence of iridium in the +III oxidation state within the material. For pure iridium oxide (IrO2 or IrOx), the transition from the amorphous to the crystalline state takes place around 400-450 °C under air - it is also in this temperature range for the transition from a mixed +III/+IV oxidation state to a +IV oxidation state. It is therefore impossible to decorrelate the influence of these 2 parameters on the electrocatalytic activity of pure IrO2 and to determine which one is more important.
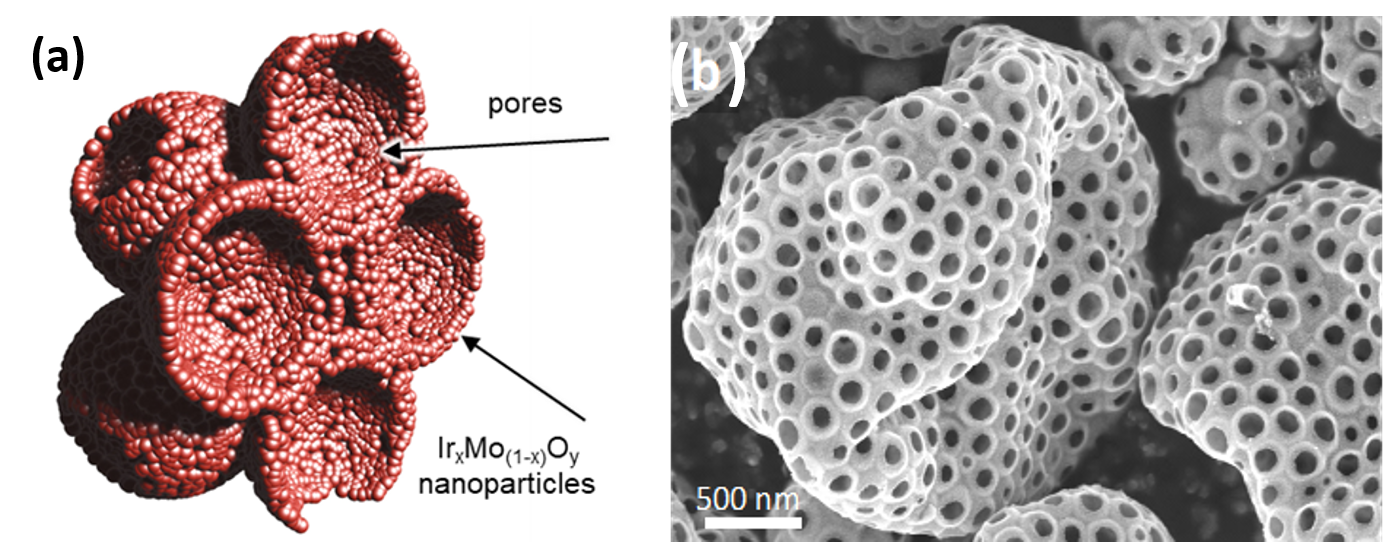
Inspired by their previous work on pure IrO2, researchers from ITODYS (University of Paris) and LCMCP (Sorbonne University) have produced a series of mixed oxides IrMOx (with M = Ti, Co, Mn, Mo), via an aerosol process, in order to obtain efficient catalysts while decreasing the amount of iridium involved. These mixed oxides are in the form of ultra-porous micron spheres whose walls are made of nanoparticles (fig. 1).
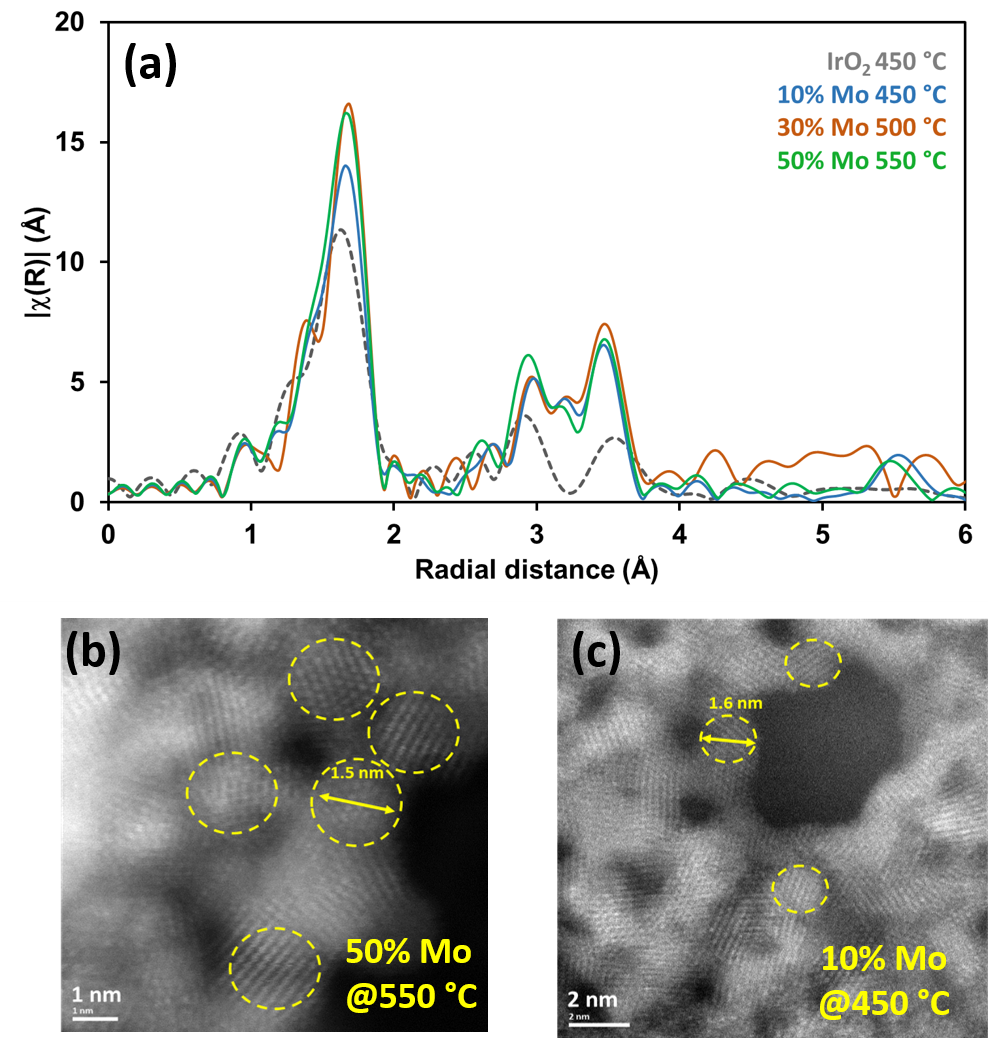
In the case of IrMoOx mixed oxides, the presence of Mo surprisingly induces a delay of crystallization, i.e. the temperature of crystalline-amorphous transition increases with the Mo content, beyond the 400-450°C range (fig. 2a). The electrochemical characterizations carried out in collaboration with the LCM of the Ecole Polytechnique (fig. 2b) indicate, moreover, that for a given Mo composition, the maximum activity corresponds precisely to the sample calcined at this transition temperature.
The analyses of the XANES spectra carried out on the SAMBA beamline ex situ and in operando mode show that, whatever the percentage of Mo, the change in the oxidation state of iridium is decorrelated from its crystalline-amorphous transition temperature and occurs systematically between 400 and 450 °C. The study of EXAFS spectra showed that this activity maximum corresponds to a state of the material obtained just after the transition temperature, i.e. to a short-range ordered material, with crystallites smaller than 2 nm in size (Figure 3).
This study combining several approaches and techniques contributes to the debate on the origin of the activity of "pseudo amorphous" IrO2 by highlighting the importance of its crystalline state among its oxidation state. This is a very important advance in the design of new efficient and stable catalysts for the OER reaction in PEM electrolyzers.