Innovative anticancer drugs: Discovery of new inhibitors for MDM2, involved in cell proliferation
The immortality of cancer cells associated with loss of life/death control mechanisms such as the regulation of apoptosis (programmed cell death), is a common stage in tumours. Mitochondria play an important role in regulating apoptosis by controlling mitochondrial membrane permeabilization. This process is mediated by the opening of the mitochondrial permeability transition pore (MPTP) and it is followed by the release of death-inducing soluble protein factors.
The immortality of cancer cells associated with loss of life/death control mechanisms such as the regulation of apoptosis (programmed cell death), is a common stage in tumours. Mitochondria play an important role in regulating apoptosis by controlling mitochondrial membrane permeabilization. This process is mediated by the opening of the mitochondrial permeability transition pore (MPTP) and it is followed by the release of death-inducing soluble protein factors.
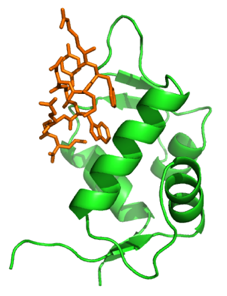
The tumour suppressor protein p53 is a transcription* (*RNA synthesis from DNA; then RNA is translated into proteins)factor that promotes cell apoptosis by the opening of the MPTP in response to stress. Another protein, called Murine Double Minute 2 (MDM2), inhibits p53 by binding to its transactivation domain and hampering transcription activation (Figure 1).
Overexpression (high production)of MDM2 is well known to inhibit the p53 pathway and to lead to uncontrolled cell proliferation and cancer. Therefore, small molecules that act as MDM2 antagonists, block the p53 recognition interface on MDM2 and restore the p53 pathway, represent an attractive approach for cancer therapy.
Over the past years a strong effort has been put worldwide to develop small molecules MDM2 antagonists.
A large number of compounds that strongly bind to MDM2 and effectively kill cancer cells in vitroand in vivohas been discovered, one of the most promising was Nutlin3a. However, the toxicity of these molecules steadily limits their application in clinics.
Starting from a virtual screening of a home chemical library, the group of Prof. Rossello at the Pharmacy Department of Pisa University (Italy) developed new classes of small molecules that act as strong MDM2 antagonists. In addition, these compounds display better biopharmacological properties compared to reference anticancer drugs such as Nutlin. However, no structural information is available to date to elucidate their mechanism at atomic level.
The collaboration between Prof. Rossello’s group and the Beamline Proxima1 at the SOLEIL Synchrotron, in Saint Aubin (France), aims at determining the X-ray crystal structure of MDM2 bound to several new inhibitors. These structures will shed a light onto the molecular mechanism underlying MDM2 inhibition and they will guide the design of more efficient and selective drugs.
Research Team:
Armando Rossello, Elisabetta Orlandini, Elisa Nuti, Susanna Nencetti, Caterina Camodeca, Doretta Cuffaro ("ProInLab", Departement. of Pharmacy, University of Pisa (UniPi))
Carole Fruchart-Gaillard (CEA, Institut des sciences du vivant Frédéric Joliot, Université Paris-Saclay, Service d’Ingénierie Moléculaire des Protéines)
Lidia Ciccone (PROXIMA-2A beamline, Synchrotron SOLEIL)
Serena Sirigu (PROXIMA-1 beamline, Synchrotron SOLEIL)