Huntington’s disease - The structure of inclusions in patients’ brains revealed by IR microspectroscopy
Huntington's disease is a neurodegenerative disease characterized by the formation of protein aggregates (inclusions) in certain brain regions. The secondary structure of these protein aggregates is still unknown, although this structure is suspected of playing a major role in the disease. Researchers at Paris Descartes University/CNRS, in collaboration with scientists from the SMIS beamline, have used synchrotron radiation infrared microspectroscopy to characterize protein aggregates in post-mortem brain tissue. This study has revealed that the structure of the aggregates varies depending on their regional and subcellular localization, and also depends on whether the disease is juvenile or adult.
A neurodegenerative disease with protein aggregates
Huntington’s disease (HD) affects 6,000 people in France. It is a neurodegenerative disease combining movement and behavioral disorders as well as dementia. The disease results from a mutation in a gene encoding a protein essential to the functioning of the human body, called huntingtin. This protein contains a repeat of the amino acid, glutamine (Q), which thus forms a long chain (polyQ). This chain has 20-35 glutamines in healthy individuals. In patients with the adult form of HD, there are 36 to 60 glutamines. This number exceeds 60 residues in those with the much rarer and more severe juvenile form. The disease is characterized by a progressive degeneration of the corpus striatum or "striped body" (the nerve structure beneath the cortex), but also of the cortex, where inclusions are formed. These are located mainly in the cell cytoplasm in adult cases and in cell nuclei in juvenile cases.
Importance of the secondary structure of protein aggregates
Recent data have shown that the secondary structure adopted by the proteins in polyQ aggregates is influenced by the experimental conditions, and that this structure plays a crucial role in cell toxicity. Thus, a simple change in temperature can alter both the structure of a polyQ peptide and its cytotoxicity. However, the structure of the aggregates that form in the brain of patients is still unknown, and there is no evidence that the aggregates formed in vitro mimic the actual structure of the aggregates in patients. To understand this disease it is therefore extremely important to characterize the structure of the aggregates present in the brain of patients.
Analysis of the secondary structure of protein inclusions using synchrotron infrared microspectroscopy
To achieve this, Paris Descartes University/CNRS researchers, together with SOLEIL scientists, analyzed samples from the brain of patients with HD using infrared microspectroscopy (IR) synchrotron radiation on the SMIS beamline. Due to the small size of the inclusions (a few microns), the highly sensitive IR synchrotron source proved indispensable. Researchers obtained IR spectra of different types of inclusions and their control compartments (cytoplasm and nuclei). They then focused on the amide I band (1600-1720 cm-1), a spectral region very sensitive to the secondary structure of proteins. Analysis of the differences between the spectra of the inclusions and controls revealed the structural characteristics of the inclusions.
Structural polymorphism of inclusions and the link to neuronal degeneration
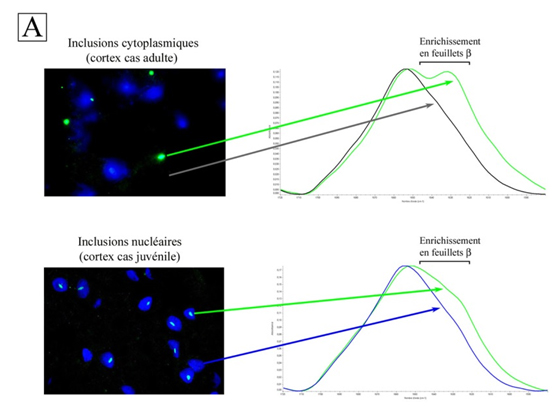
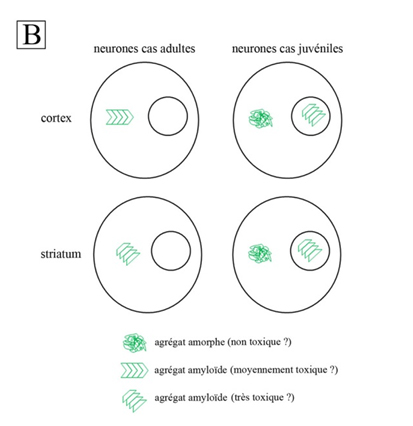
Thus, the cytoplasmic inclusions of the cortex and corpus striatum of adult patients are both highly enriched with b sheets, indicating an amyloid structure1; the IR spectra also show differences between cortex and corpus striatum (Fig. 1A). Their amyloid structures are different. The fact that the degeneration is more pronounced in the corpus striatum than in the cortex of adult cases suggests that the amyloid structure of inclusions in the corpus striatum is more toxic to neurons than that found in cortex. As for juvenile cases, their nuclear inclusions have an amyloid structure comparable to those of the corpus striatum of adult cases, while their cytoplasmic inclusions are amorphous aggregates without amyloid structure. This may explain why nuclear inclusions are toxic in juvenile patients, while cytoplasmic inclusions are harmless.
Conclusion
This paper describes a complex variety of aggregation (Fig. 1B) and reinforces the hypothesis that there are links between the structure of aggregates and neuronal toxicity. The researchers will now verify experimentally the toxicity of amyloid structures described in this study and seek to understand the mechanisms of toxicity. The flexibility of protein aggregates could, for example, depend on their amyloid structure, thus affecting the exposure of polyQ on their surface. This latter might result in abnormal interactions with cell components, disrupting their activity and leading to cell death.
1 – Amyloid: this initially meant "starch-like." The histo-pathological definition was later widened to include biophysical techniques. According to this latest definition, amyloid fibers are characterized using X-ray diffraction as having a b-sheet structure.
Infrared microspectroscopy (which has a lower resolution than X-ray diffraction), also distinguishes amyloid fibers from other protein fibers by their b-sheet content. The IR spectral signature of amyloid fibers is the signal between 1620 and 1630 cm-1 in the amide I band. An increase in intensity is seen as evidence of the presence of amyloid protein aggregate.