Dirac electrons in blue phosphorene monolayer
Physicists have for the first time synthesized and characterized a blue phosphorene sheet with a metallic-like electronic structure and thus discovered a new two-dimensional material with properties common to graphene.
Graphene, the first known 2D material with its carbon atoms assembled in a single layer in a "honeycomb" pattern, has a peculiar electronic structure. If we draw the relation between the energy of the graphene electrons and their momentum (also called dispersion relation), we do not obtain the characteristic curves of classical materials but a cone, called Dirac cone, corresponding to the fact that the energy of the electrons is proportional to their momentum. Dirac electrons at the Fermi level have interesting properties: everything happens as if their mass was zero, giving them the possibility to travel at a constant speed, like light. This property explains why graphene is an exceptional electronic conductor.
After the discovery of graphene, the scientific community started looking for new two-dimensional materials. This is how blue phosphorene, made of phosphorus atoms, was recently synthesized. It has an atomic structure similar to that of graphene but slightly corrugated, like corrugated metal. If graphene is metallic, the blue phosphorene synthesized so far behaves like a semiconductor and finds applications for new electronic devices.
Three teams from the Saclay plateau, from the Orsay Institute of Molecular Sciences (ISMO, CNRS / Université Paris-Saclay), the SOLEIL Synchrotron (SOLEIL, CNRS) and the Condensed State Physics Service (SPEC, CEA / CNRS), in collaboration with a team from the Central University of Florida and a team from the Mohammed 5 University in Morocco, have succeeded, for the first time, in synthesizing a monolayer of blue phosphorene, this time with a metallic electronic structure similar to that found in graphene.
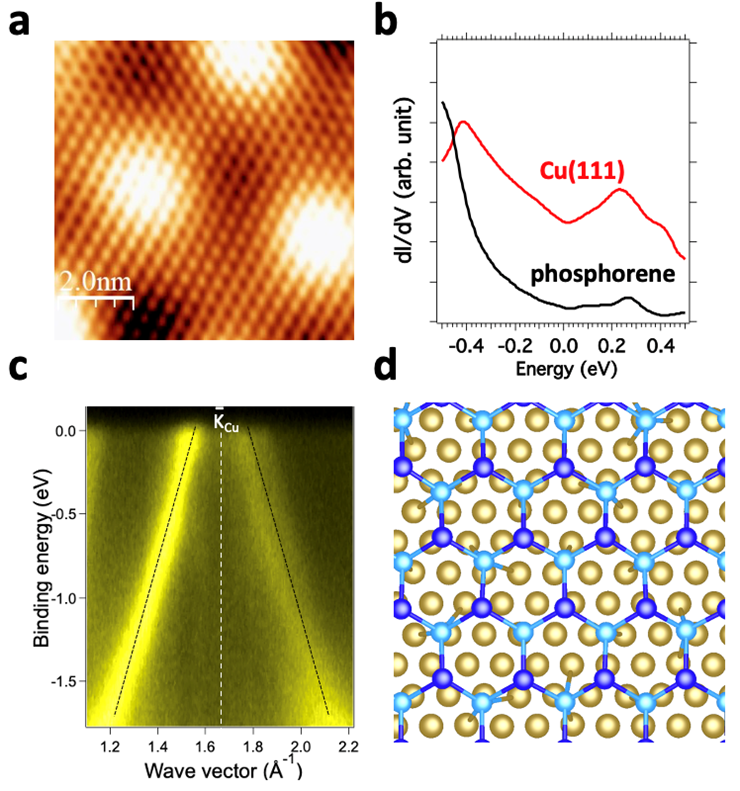
The researchers deposited phosphorus atoms on (111) oriented copper crystal by evaporation under ultra-high vacuum. They were able to identify the atomic and electronic structure of a blue phosphorene sheet by combining state-of-the-art analysis tools such as scanning tunneling microscopy and spectroscopy (STM-STS), Low-energy electron diffraction (LEED), photoelectron spectroscopy (PES) and angle-resolved photoemission spectroscopy (ARPES) on the TEMPO beamline at SOLEIL.
Microscopy and tunneling spectroscopy have shown that the blue phosphorene sheet is highly ordered and that its natural corrugation is reduced by the interaction with the copper substrate, which brings it closer to the natural shape of graphene. Tunneling spectroscopy also reveals a metallic character, while ARPES has identified the electronic states characteristic of a Dirac cone (Figure 1). This cone constitutes, as for graphene, a clear and unmistakable signature of the presence of Dirac electrons within the blue phosphorene sheet. Furthermore, a natural formation of self-organized phosphorus nanostructures on the blue phosphorene sheet induces an opening of a band gap in the linear dispersion forming the Dirac cone, thus demonstrating that the Dirac electrons are scattered by the network formed by these nanostructures.
Density Functional Theory (DFT) calculations have confirmed the atomic structure and the metallic character of the blue phosphorene sheet.
This discovery, which calls for further studies on the Dirac properties of blue phosphorene, opens fascinating perspectives from both a fundamental and an applied point of view in the study of a new two-dimensional versatile material with a Dirac cone.
a) STM image showing the structure of the blue phosphorene sheet deposited on copper.
b) dI/dV curves corresponding to the density of electronic states of the clean copper surface and after deposition of the blue phosphorene sheet. These curves show that the phosphorene has a metallic character since the corresponding dI/dV does not reveal an electronic gap (non-zero electron density).
c) ARPES figure after deposition of blue phosphorene, showing two linear dispersions in the form of a Dirac cone, signature of the presence of Dirac electrons typical of those found in graphene.
d) Atomic model of the blue phosphorene sheet defined from the STM image in a) and used for DFT calculations. The dark blue (first layer) and light blue (second layer) atoms correspond to phosphorene and the yellow atoms correspond to copper.