Deep photodynamic therapy: a nanoflash-light to activate drugs in solid tumors
Although light has been used in medicine for decades, until now, researchers faced a major limitation: only the reachable zones can be treated (skin, esophagus, lungs…). Scientists from SOLEIL Synchrotron and the Center of Molecular Biophysics of Orléans designed a photochemotherapy method ingeniously using lanthanide-based micellar structures, which allows solving this issue. Their work was published in the Nano Research Review, and offers numerous perspectives, in magnetic resonance imaging as well.
In order to treat some tumors, light can prove very effective, particularly thank to photodynamic therapy (PDT). This method relies on the use of substances which become toxic for their immediate environment when subjected to visible light. The whole issue is about vectorising these substances in the tumor before illuminating them to trigger the reaction.
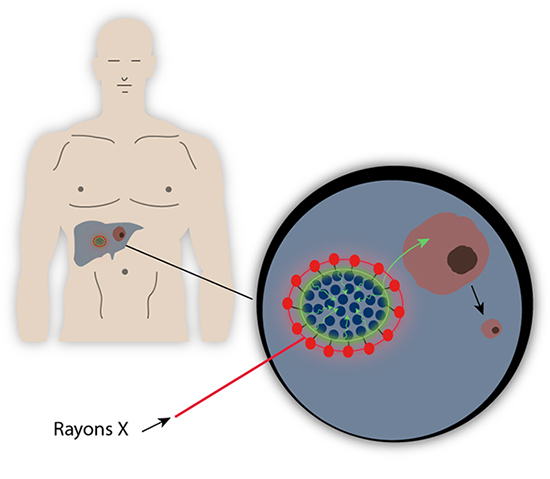
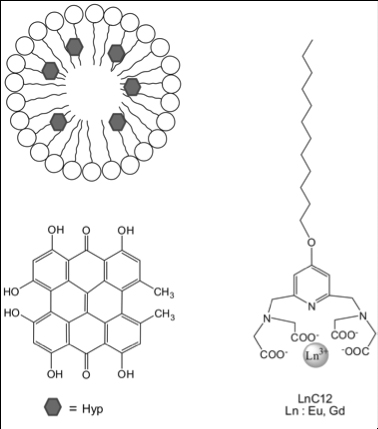
Scientists had the idea to trigger a chain reaction allowing them to access deeper tumors. First of all, a liponanoparticle micellar structure, with peripheral lanthanides, comes flanking a photosensitizer, the hypericin, which allows vectorising this highly hydrophobic substance until it reaches the zone to be treated. Then, the whole area is irradiated by X-rays that initiate the chain reaction. Actually, lanthanides present a long-known luminescence property, and thus have enhanced X-rays detection systems in the 90s. When submitted to X-rays, lanthanides such as Gadolinium or Europium reemit ultraviolet or visible light. This secondary light source then irradiates the central hypericin which will produce reactive oxygen species (ROS), toxic substances that will kill the surrounding tumor cells.
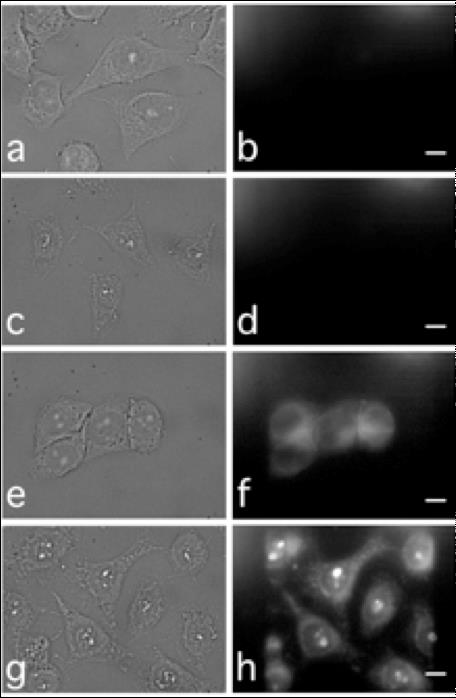
Practically, scientists used the METROLOGIE and DISCO beamlines of SOLEIL in order to irradiate liponanoparticles and collect the associated luminescence spectra. They thus showed that the presence of lanthanides did indeed allow emitting a UV-visible radiation which matches the absorption spectrum of hypericin. They then characterized the light energy transfer between those two molecules. They later measured with mass spectrometry the increasing of production of reactive oxygen species, consequence of the light absorption of hypericin. Furthermore, the UV-luminescence properties of the micellar structure allowed the scientists to monitor them inside the cells, and even inside the nuclei.
The system proposed here benefits from being extremely versatile: it is indeed possible to enlarge this method to other photosensitizers, or other liponanoparticles already tailored for specific targets. When current photodynamic treatments amount to no more than superficial therapies, the use of the penetrating capacity of X-rays promises to bring new perspectives in the issue of treating deep tumors.