The crystallographic structure of the enzyme PolD suggests a new paradigm for classification of DNA polymerases
In all forms of cellular life, the enzymes DNA polymerases (DNAPs) play a central role in the replication, maintenance and repair of the genome. Consequently, the DNAPs have been the subject of intense research for many decades. Thanks to data collected using the PROXIMA-1 and PROXIMA-2A beamlines, teams at the Pasteur Institute and IFREMER have been able to resolve the structure of an archaeal DNAP. Their results show that while this enzyme is atypical amongst the different families of DNAPs, by contrast if shares some characteristics with RNA polymerases. The results are published in Nature Communications.
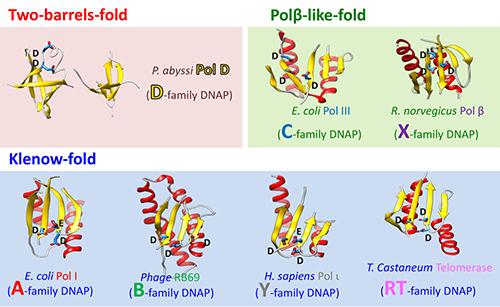
The DNAPs have been grouped into seven different families based on the alignments of their amino acids sequences: PolA, PolB, PolC, PolD, PolX, PolY and reverse transcriptase (RT). It should be noted that nearly all of the DNAPs can be grouped into two structural superfamilies: Klenow type folding (PolA, PolB, PolY and RT) and Polβ type folding (PolC and PolX). The last family of DNAPs the structure of which remains unknown and whose catalytic domain has no associated folding is PolD.
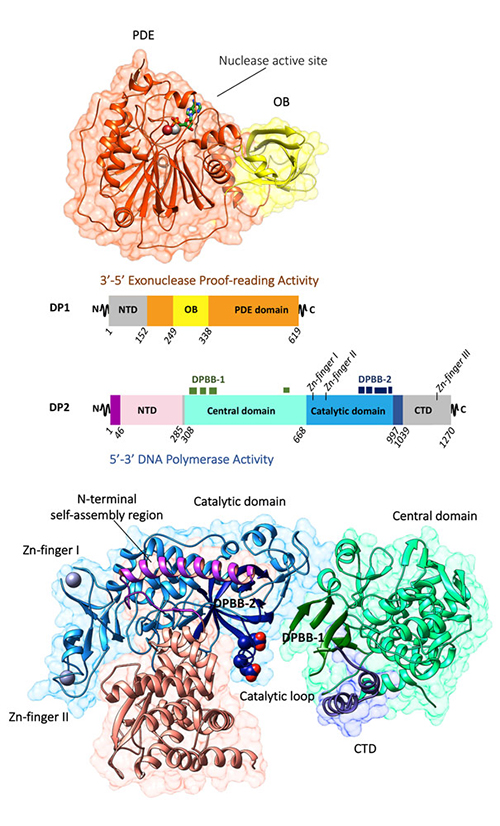
PolD is present in all Archaea*, with the exception of crenarchaeota. It is a replicative DNAP responsible for initiating the reproduction of the DNA. It is comprised of a large subunit (DP2) that supports the 5'-3' polymerase activity and a small subunit (DP1) supporting the 3'-5' exonuclease activity responsible for proofreading. Apart from the N-terminal regions of the subunits DP1 (1-50) and DP2 (50-280), the structures of the polymerase and exonuclease catalytic domains remain unknown.
To determine the evolutionary origin of the DNAPs of the D family, scientists have resolved the crystallographic structure of two large structures of the subunits DP1 and DP2 of the PolD of the archaeon Pyrococcus abyssi. Each of the structures has been determined individually by experimental phasing using SAD on the beamlines PROXIMA-1 and PROXIMA-2A, for DP2 and DP1 respectively (Figure 1).
The structures of subunits DP1 and DP2 reveal that the PolD is an atypical DNAP. Firstly, the exonuclease proofreading DP1 subunit has phosphodiesterase folding which is radically different to the dnaQ-like domains found on the large majority of other DNAPs. Secondly, the DP2 subunit exhibits no structural similarity with other known DNAPs. With the structure of DP2, a third class of DNAPs has been created (Figure 2).
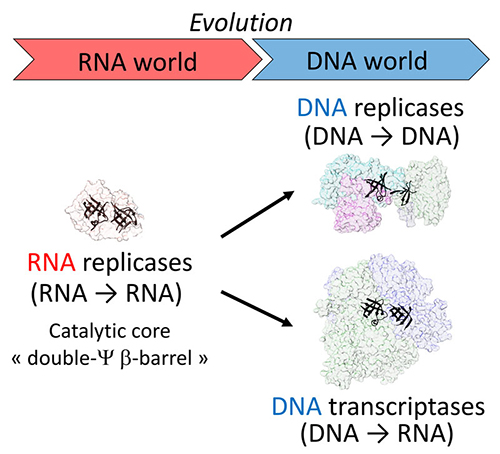
Unexpectedly, scientists have shown that DP2 shares a structural homology with the "double beta-barrel" folding of the catalytic core of the RNA polymerases (RNAPs), including the multisubunit transcriptases in the three domains of living organisms. This unexpected homology defines a new paradigm for the classification of DNAPs and RNAPs, as well as a possible common evolutionary origin between the different types of DNAPs and RNAPs. This links for the first time, excluding the world of viruses, replication and transcription of DNA within a single common protein superfamily. The fact that PolD and "double beta-barrel" RNAPs share a common catalytic core suggests that the protein mechanisms responsible for the replication and transcription of DNA evolved at the same time.
Indeed, this study suggests that the "double beta-barrel" catalytic core could have facilitated the transition from an RNA world to a DNA world, by allowing the joint evolution of replication and transcription based on a common catalytic core, rather than the two types of enzymes having have been "invented" independently (Figure 3).
* Archaea: unicellular organisms without a nucleus, have for several decades been classified in a different domain to bacteria.