Controlled and targeted chemical reactions, thanks to the water surface
In modern chemistry the control, for a given reaction, of the precise location where it will take place on the molecules involved is a challenging task but is crucial because it may open up new avenues in synthetic, biological, and materials chemistry. For example, formation of 2D polymers could enhance the functionality of semiconductor devices. Such precision, known as selectivity, involves guiding a reactant molecule to a specific position to favor the formation of one product over another. Scientists from German laboratories have demonstrated a highly promising protocol for controlling "topological" selectivity, or site-selectivity, which is particularly demanding.
In chemistry, selectivity is categorized into three types: chemo-selectivity (preference for one functional group over another), diastereo-selectivity and enantio-selectivity (favoring the formation of one of the diastereoisomers or enantiomers* of a molecule), and regio-selectivity or site-selectivity (preference for a specific location in a molecule). To control site-selectivity, chemists have explored various strategies, such as designing selective catalysts, creating alternative reaction pathways, using protective chemical groups, and introducing structural hindrances.
Regarding site-selectivity, teams of scientists from Germany have developed a method to make certain chemical reactions occur at specific sites. This innovative approach, conducted on the water surface, opens up possibilities for diverse bond formations.
Using a charged surfactant monolayer, they manipulated the environment where reactions take place. This caused molecules to behave differently on the water surface than in the bulk, leading to a distinctive packing structure of reagent molecules.
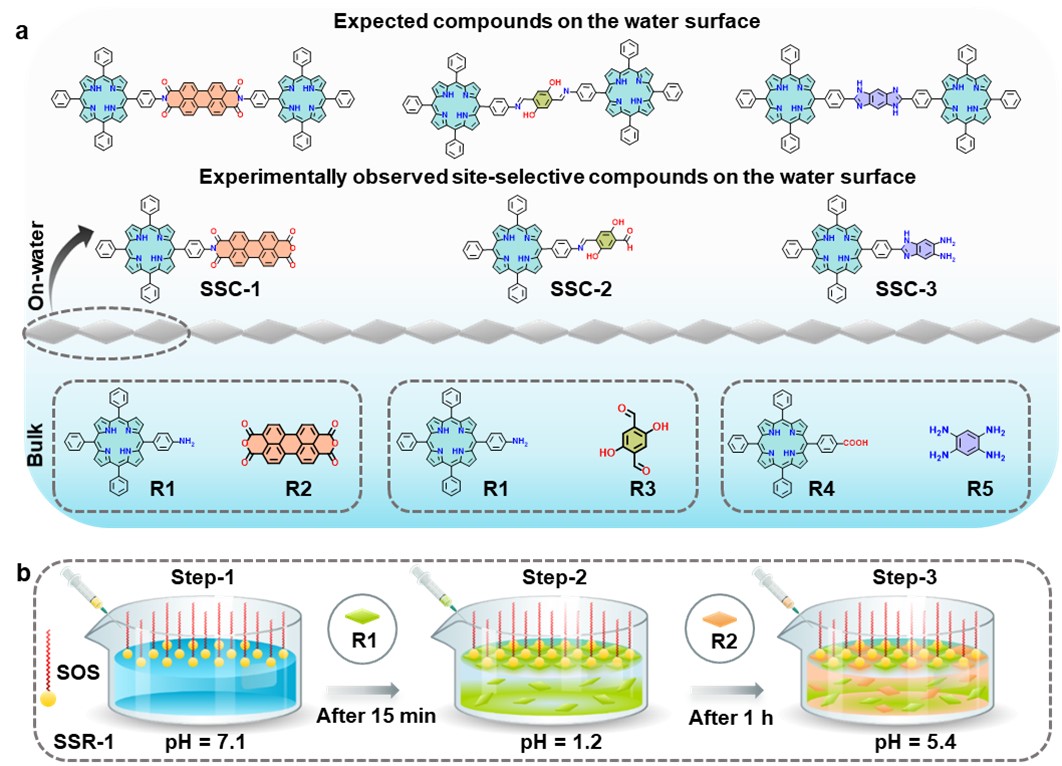
(a) Schematic of experimentally observed site-selective and expected compounds on the water surface.
(b) Stepwise schematic representation of site-selective chemical synthesis approach on the water surface (see text).
The approach (see Figure 1) consists in an initial formation of a crystalline surfactant monolayer (here Sodium Oleyl Sulfate, SOS) adsorbed on the water surface (step I; the surfactant's hydrophilic chemical groups point to the water surface). In a second step (step II), the first reagent R1 (an amino-substituted porphyrin) is added into the water. The pre-adsorbed surfactant layer guides the assembly of R1 molecules into a well-defined structure adsorbed below the surfactant monolayer where the reactive groups are properly aligned. In step III, a second reagent R2 (perylenetetracarboxylic dianhydride) is injected in the water that induces the formation of chemical links R1-R2 within the pre-constrained R1 structure. This structured arrangement allowed precise control over the formation of specific bonds, ensuring the creation of one-sided bonds (SSC-1 in fig.1) instead of undesired two-sided products. Similarly, site-selective reactions on the water surface were further demonstrated by three reversible and irreversible chemical reactions, such as those forming imide (SSC-1), imine (SSC-2), and 1,3-diazole (imidazole) bonds (SSC-3) involving porphyrin molecules (Fig. 1).
Delving into the intricate world of molecular assembly on water surface, this study utilized cutting-edge techniques to gain a comprehensive understanding of the sequential assembly process: sum frequency generation spectroscopy and X-ray diffraction, along with theoretical calculations. In particular, the in-situ grazing incidence X-ray diffraction (GIXD) measurements at the air/water interface on SIRIUS beamline at SOLEIL, played a pivotal role in unraveling the structural nuances at each step.
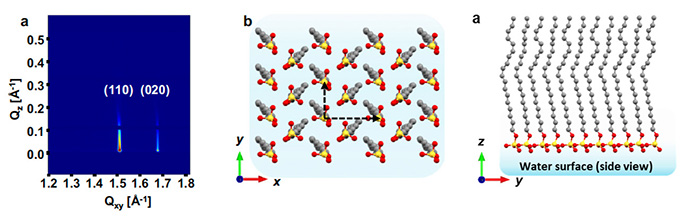
The initial formation of the crystalline surfactant monolayer on the water surface, as captured by GIXD, revealed a well-defined lattice structure that matched well with the 2D lattice structure of the SOS surfactant monolayer derived from theoretical modeling (Figure 2).
a - In-situ grazing incidence X-ray diffraction (GIXD) pattern diffraction of the SOS surfactant monolayer.
b & c - 2D lattice structure of the SOS surfactant monolayer deduced from GIXD experiment and simulations.
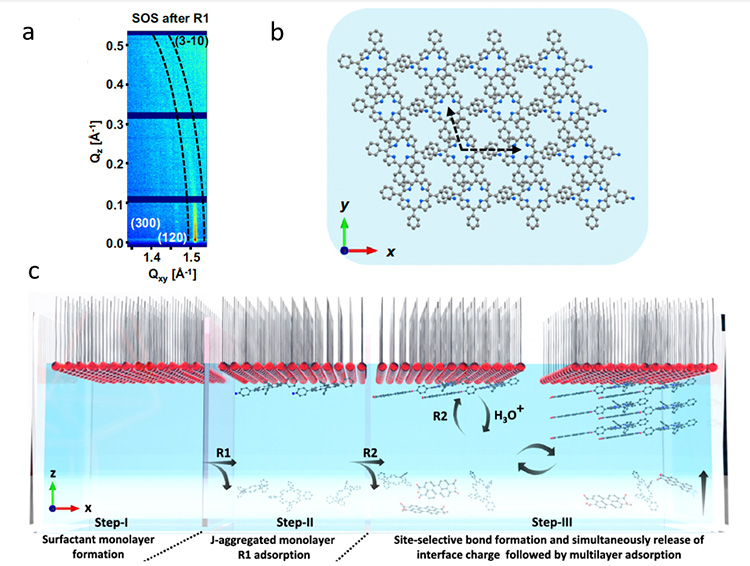
Moving forward, the study explored the pre-organization of R1 molecules beneath the surfactant monolayer, revealing a distinctive 2D-confinement of R1 in J-aggregated** packing guided by the surfactant monolayer (Figure 3).
a - In-situ grazing incidence X-ray diffraction (GIXD) pattern diffraction after the injection of R1 (Step II).
b - Lattice structure of the R1 monolayer on the water surface c schematic illustration of the dynamic interface showing the stepwise mechanism for the multilayer growth of the site-selective product on the water surface
The in-situ GIXD experiments have played a major role in enabling the precise characterization of structural relationships between the SOS surfactant monolayer and the R1 assembly. The level of detail provided marks a significant contribution, shedding light on the intricacies of molecular arrangements crucial for advancing our understanding of controlled chemical reactions on water surface. This research opens doors to controlled and targeted chemical reactions with potential applications in various fields for designing functional materials and biomimetic systems.
* Diastereomers and enantiomers: 2 types of isomers - i.e. molecules with the same atomic composition, but not the same structure - where the difference lies in the spatial arrangement of their atoms or groups of atoms.
** J-type aggregation: one of the configurations that 2D molecules can adopt when they aggregate, see here.