Contamination of groundwater – Can calcite trap arsenic?
Arsenic (As) contamination of groundwater is a very serious environmental health threat in many countries all over the world. A better understanding of the mechanism of As uptake and release by rock minerals can lead remediation strategies. But minerals like calcite, ubiquitous in the Earth’s crust, are difficult to study because of their complex and inhomogeneous structure. For this purpose, the micro X-ray absorption spectroscopy technique using synchrotron radiation is particularly relevant.
Arsenic, a poison
Arsenic is a semi-metal element in the periodic table. It is odorless and tasteless and it enters drinking water supplies from natural deposits in the earth or from agricultural and industrial practices. When As contaminated groundwater is used as drinking water, it constitutes a hazard to the health of the consumers. Prolonged exposition to arsenic can include thickening and discoloration of the skin, stomach pain, nausea, vomiting; diarrhea; numbness in hands and feet; partial paralysis; and blindness. Arsenic is also considered carcinogenic and has been linked to cancer of the bladder, lungs, skin, kidney, nasal passages, liver, and prostate. The major source of contamination is not anthropogenic but geogenic, i.e. due to the erosion or dissolution of arsenic-bearing rock minerals and the consequent release of amounts of arsenic up to thousands of mg/L (the limit concentration set by the US agency for the protection of environment (EPA) being of 0.01 mg/L). Therefore it is of importance to know where As-rich groundwater occurs so that actions can be taken to prevent health issues to the population. South East Asia, is the region that is most severely affected by natural arsenic contamination in groundwaters, the threat being particularly serious in Bangladesh, Nepal, Vietnam, and China. Nevertheless, also in Europe areas of highly enriched As groundwater exist, albeit at a more regional scale.
High As contaminated groundwaters were found in the Pannonian bassin (Hungary, Romania, Croatia and Serbia), but also in Spain, Italy, Greece, and France (e.g. Cézallier area, Massif Central; ref.1), and no reports on As in groundwater are available for many other European countries to date.
How to study the arsenic trapping by minerals
Beside the localization of risk areas, it is also of fundamental importance to understand the mechanism of As uptake and release by rock minerals, since such knowledge can lead remediation strategies. Many natural minerals or engineered materials can uptake considerable amounts of arsenic. Because of the ubiquity of this mineral in the Earth’s crust and its stability in a variety of geologic environments, calcite (CaCO3) can play an important role in limiting the mobility of As.
Nevertheless, while several studies were performed on synthetic calcite, the efficiency of natural calcite to act as a mineralogical trap for As has not been extensively studied, also because of the inherent difficulties of dealing with complex, inhomogeneous matrixes. In this framework, synchrotron radiation techniques were demonstrated to be extremely useful. In particular, high resolution elemental distribution maps can reveal the association of As with other mineral phases present in the natural calcite samples (mainly Fe and Mn oxyhydroxides), while micro X-ray absorption spectroscopy (XAS) reveals the oxidation state of As at point of interests determined from the elemental distribution maps.
The relevance of micro X-ray absorption spectroscopy
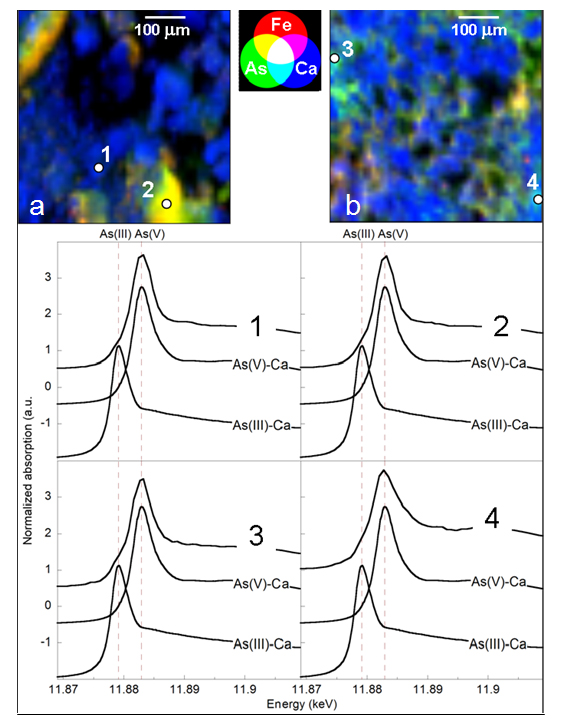
Portions of natural calcite samples, i.e. As rich lacustrine travertines, from the Pecora River valley (Italy) were prepared as polished thin sections and studied at the DiffAbs beamline acquiring several 500x500 mm elemental distribution maps at a resolution of 10 mm (Figure 1, top panels). In addition, several X-ray Near Edge Spectroscopy (XANES) spectra were acquired on point of interests on the above maps (Figure 1, bottom panel). The results of this work confirm and support the results obtained in previous works (ref.2,3,4) using synchrotron radiation bulk XAS and other techniques. In particular, the present experiment allowed to confirm that in the natural travertines studied As(III) is incorporated in the calcite lattice by the arsenite-carbonate substitution mechanism suggested by Román-Ross et al. (ref.5), although this substitution occurs only for a small fraction of the total amount of As present in these travertines. As incorporation by calcite may become important in conditions (e.g., reducing and/or alkaline environments) where adsorption of As onto Fe-oxyhydroxides is not favored. This can have high impact from the environmental point of view, because when As is incorporated in a mineral phase, instead of adsorbed on its surface, its mobilization in the environment is highly reduced.
References :
1. Le Guern C. et al (2003) Appl. Geochem. 18, 1313–1323.
2. Di Benedetto F. et al. (2006) Earth Planet. Sci. Lett. 246, 458–465.
3. Costagliola P. et al. (2007) IMWA Symposium 2007: May 27–31 (eds. F. Frau and R. Cidu), Cagliari/Sardinia Italy, pp. 415–418.
4. Bardelli F. et al. (2011) Geochim. Cosmochim. Acta 75, 3011–3023.
5. Román-Ross G. et al. (2006) Chem. Geol. 233, 328–336.