Communication between human cells and their environment. A major advance thanks to structural biology
Cells communicate with their environment via receptors on their surface. When a signal-protein approaches these receptors, they can pass along a message to the inside of the cell, for example the instruction to grow, which can lead to tumor formation if there is an “error in the message”.
New research conducted by an international team, and involving the PROXIMA-2A and SWING beamlines, reveals the 3D structure of the human ALK receptor, which is implicated in various cancers and other diseases (such as lupus and obesity). These structural insights, published in the journal Nature, will lead to a proper understanding of the function of these receptors, the first important step towards therapeutic approaches.
Cells communicate with their environment via signal-proteins that attach to receptors on the cell surface. Think of these receptors as communication antennae that can only receive specifically encoded signals. When an appropriate signal from outside the cell – in the form of a specific protein for the receptor, such as a cytokine – approaches an antenna, the signal will be communicated to the inside of the cell. This can initiate many important cellular processes, such as cell growth and division.
When the communication between such proteins and receptors becomes uncontrolled, excessive, or interrupted, these dysfunctions can lead to severe diseases such as cancer, or inflammatory and autoimmune disorders. A group of receptors central to human health is known as the Receptor Tyrosine Kinases (RTKs). Humans have about 58 of these RTKs organized into 20 families. Functional defects in these receptors are relevant for several cancers, autoimmune, neurological diseases and metabolic disorders.
ALK family
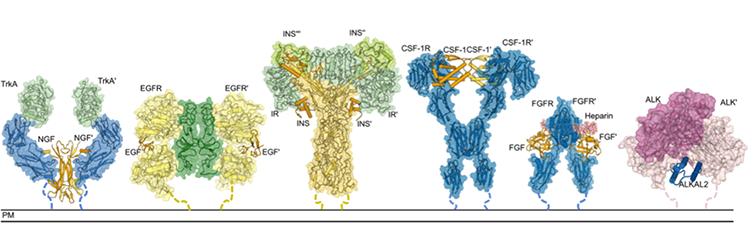
Due to their critical roles in physiology and disease, most RTKs have been well-researched, but for one group, the Anaplastic Lymphoma Kinase (ALK) family, information is sorely lacking. These receptors have been known to exist for over three decades, and yet structural information about how they interact with their signaling proteins has remained a question mark – until now. This new study, spearheaded by the PhD student Steven De Munck from the team of Prof. Savvas Savvides (VIB-UGent Center for Inflammation Research, Belgium), the National Cancer Research Institute (Tokyo, Japan), the Memorial Sloan Kettering Cancer Center (New York, USA), and ħ Bioconsulting LLC (Minnesota, USA) has now revealed the 3D structure of ALK and LTK (Leukocyte receptor tyrosine kinase), another RTK, as well as their structures when bound to their signal-proteins (i.e. cytokines). These 3D structures provide critical information for understanding the function of these receptors.
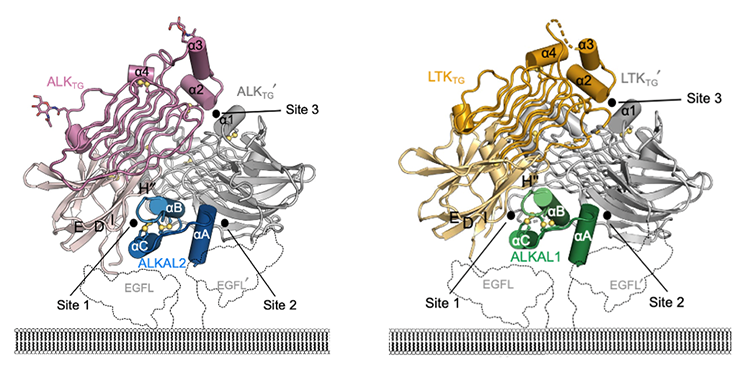
The signaling cytokine is represented in blue in the complex with ALK and in green with LTK. The ALK and LTK receptors dimerize when they bind the cytokine molecule: the respective complexes contain 2 ALK molecules (in pink and white) and 2 LTK molecules (in yellow and white).
Only the part of the receptors that is external to the cell -the same part that binds the signal molecules coming from the environment- is represented here. It resides above the double layer of lipids constituting the cell membrane; the black dotted line depicts the transmembrane part of the receptors, which "anchors" the receptors to the cell and transmits the signal inside the cell.
Critical X-ray diffraction data for the two receptor-cytokine complexes were obtained at SOLEIL on the beamline PROXIMA-2A, and the technical support provided by the beamline team was of paramount importance to achieve these breakthrough findings. Furthermore, Small angle X-ray scattering (SAXS) experiments were conducted at SWING beamline.
The scientific team was surprised by how unique and unprecedented these proteins and their assemblies are, illustrating how basic research is essential for uncovering novel concepts and mechanisms in disease-causing processes.
ALK and its mutated versions are involved in, for example, the pediatric cancer neuroblastoma, colon cancer, melanoma, and possibly even obesity. However, the lack of structural insight has prevented the specific therapeutic targeting of ALK receptors. These new results are therefore very promising for future therapeutic developments.