Capturing Catalysis in Tiny Cages: Green Chemistry for a Sustainable World
Polyoxometalates (POMs) are a fascinating class of metal-oxo* clusters known for their versatile applications in catalysis, energy storage, and environmental applications. Despite their promise, many POMs remain underutilized due to their insolubility or instability in aqueous solutions. To address this challenge, researchers from KU Leuven and PROXIMA-2A employed a cutting-edge supramolecular strategy to synthesize and stabilize POMs using coordination cages. Their findings represent a significant leap forward in the field of green and sustainable chemistry.
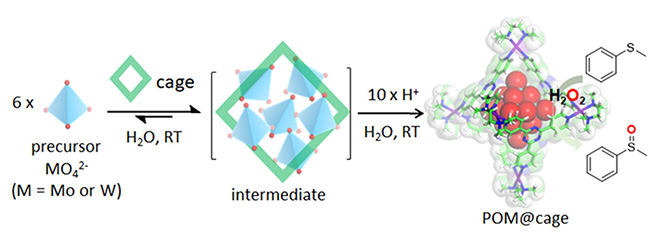
The stability and catalytic efficiency of POMs in solutions are determined by complex interactions at the molecular level. POMs such as the Lindqvist anion [M6O19]²⁻ (M = Mo or W) are characterized by extreme hydrolytic instability, often requiring organic solvents or additives for their synthesis and applications. However, these approaches limit their utility in environmentally friendly contexts. To overcome these barriers, the researchers adopted a cavity-directed "ship-in-a-bottle" strategy, using a water-soluble coordination cage to encapsulate and stabilize POMs in aqueous solutions under mild conditions.
In this context, the coordination cage acts as a host, providing a confined microenvironment that mimics natural enzymatic systems. This approach facilitates the in-situ formation of Lindqvist POMs from simple precursors like molybdate (MoO4²⁻) and tungstate (WO4²⁻) building blocks. Once formed and encapsulated within the cage, these POMs are stabilized by an intricate network of host-guest interactions, overcoming their intrinsic instability in water. Using the microfocus X-ray beam at PROXIMA-2A, the researchers confirmed the successful formation and encapsulation of Lindqvist POMs within the cavity of the cage by single crystal X-ray diffraction. They demonstrated that this encapsulation prevents hydrolysis and enhances the solubility of POMs in water, a critical factor for their application in catalysis.
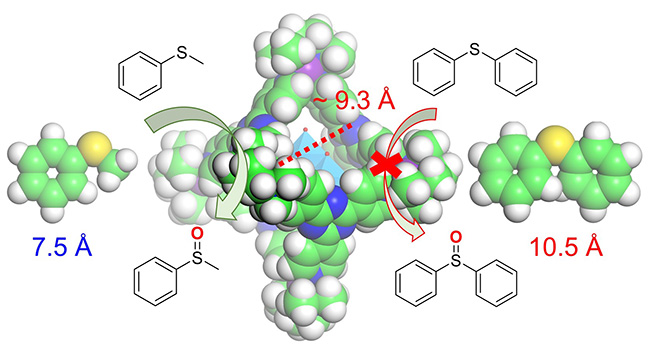
One of the most exciting outcomes of this study is the application of the POM host/guest system in catalysis. The group explored the catalytic potential of the Mo6O19@cage complex for the selective oxidation of sulfides to sulfoxides and sulfones, a reaction that is crucial in industries ranging from pharmaceuticals to fine chemicals. They achieved high yields and selectivity under mild conditions in aqueous solutions, showcasing the potential of these POM-based systems as sustainable catalysts. The study also revealed the system's remarkable substrate selectivity. For instance, the confined space of the coordination cage restricts access to larger molecules, enabling precise catalytic activity reminiscent of natural metalloenzymes. This synergy between the POM and its host cage underscores the potential of combining supramolecular chemistry with catalytic design.
This research not only advances the fundamental understanding of POM chemistry but also offers a blueprint for developing next generation of supramolecular catalysts based on POMs. By leveraging the confined spaces of coordination cages, the team demonstrated a novel method to stabilize otherwise elusive POMs, broadening their applicability in green chemistry.
The findings from this study pave the way for a paradigm shift in the synthesis and application of POMs in various domains. Future research could focus on exploring other unstable clusters, enhancing the versatility of the “ship-in-a-bottle” approach. This work highlights the transformative potential of supramolecular chemistry in addressing longstanding challenges, offering a glimpse into a more sustainable and innovative future for chemical technologies.
* oxo: in chemistry, refers to the presence of an oxygen atom doubly bonded to another atom.