Biradicals - An elusive kind of molecules
Biradicals are molecules with two unpaired electrons. The simplest and best known examples of biradicals are oxygen (O2) and ozone (O3), but other biradicals appear in combustion processes or as intermediates in chemical reactions. This immediately shows that their chemistry can be of great relevance for our daily life.
Scientists from Bordeaux, Milano, Paris and Würzburg started a project at the DESIRS beamline, aimed at elucidating the structure of biradicals with boron atoms. Their first results are published in The Journal of Physical Chemistry Letters.
The spins of biradicals can be either oriented parallel or antiparallel with respect to each other, forming either triplet or singlet biradicals. It is still difficult to accurately describe the structure of biradicals by either theory or experiment and more experimental data from high-resolution gas phase experiments are required. One of the main challenges is the generation of biradicals, because most of them are highly reactive, cannot be stored and have to be produced in situ.
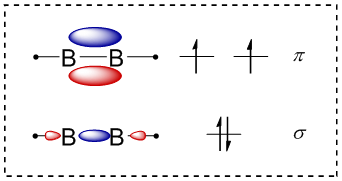
Recently a team composed of scientists from Bordeaux, Milano, Paris and Würzburg started a project at the DESIRS beamline, aimed at elucidating the structure of biradicals with boron atoms using Synchrotron radiation. They successfully generated and studied diborene (HBBH), depicted in Figure 1, a model system of great interest in inorganic chemistry.
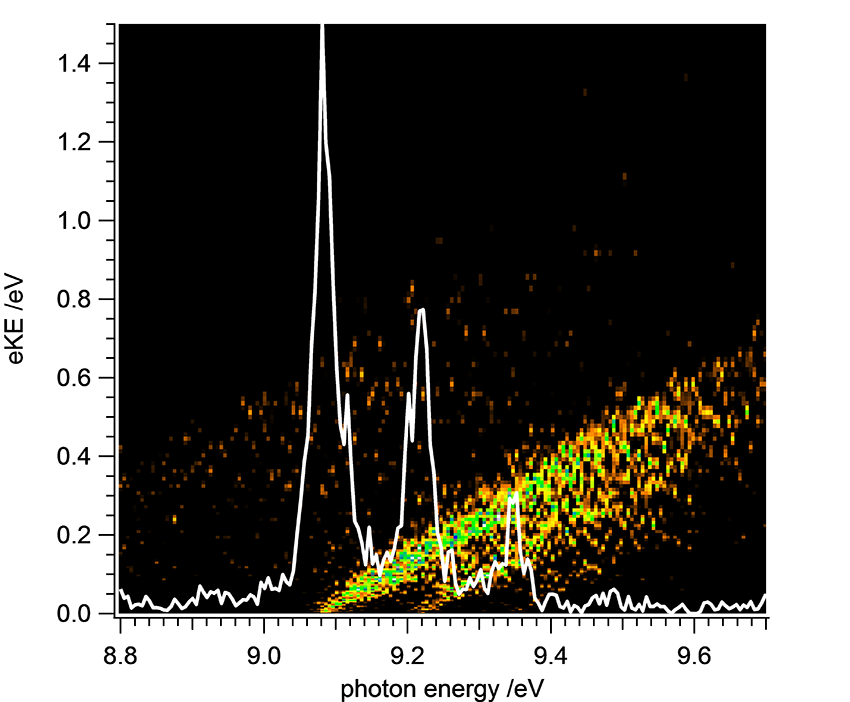
As visible in the molecular orbital diagram the molecule has one unpaired electron on each boron atom. Diborene was produced from B2H6 in a fluorine atom discharge reactor, developed by the Bordeaux-team of Jean-Christophe Loison. Fluorine atoms produced in the microwave discharge abstract successive H-atoms from B2H6, until HBBH biradicals are left. They are then ionized by Synchrotron radiation, i.e. a positively charged ion and an electron are produced. The characteristics of Synchrotron radiation and the setup at the DESIRS beamline allow to correlate the electrons with the ions, so the scientists always know from which molecule the electron is ejected. The kinetic energy of the electron (eKE) is subsequently analyzed as a function of the photon energy (energy of the Synchrotron light) and one obtains the map shown in Figure 2. Due to the law of energy conservation a spectrum (white line) of the HBBH cation is derived from this map, which can be compared to computations and finally gives detailed information on the neutral biradical and on the cation.
The structure of the spectrum is dominated by the relative motion of the boron atoms (boron-boron vibration) in the molecule. Upon ionization the bond between the two boron atoms becomes weaker and the bond length increases. This increase leads to an excitation of vibrational motion that is visible in the spectrum as a series of bands. Further details were derived from high-level computations, for example a so-called Renner-Teller splitting of the bands, which accounts for the finer details.
An interesting aspect is the possibility to exchange the hydrogen atoms (see Figure 1) to other atoms or more complex chemical groups. This is not possible in a simple biradical like O2. Photoionization using Synchrotron radiation then offers the opportunity to follow the changes in chemical bonding that are associated with structural modifications. Thus diborenes allow to systematically study the properties of biradicals as a function of the chemical structure. This line of research will be explored in the near future.