When like-charged surfaces attract each other
When a surface is immersed in water, it becomes spontaneously charged. Interactions between charged surfaces play a key role in many phenomena, such as the cohesion of cement, the behaviour of polymers, and the adhesion of living cells. Yet under certain conditions, a surprising effect occurs: two surfaces carrying the same charge can attract each other!
To better understand this counterintuitive phenomenon, researchers at the Institut Charles Sadron, in collaboration with the SIRIUS beamline, studied stacks of charged lipid layers.
X-ray reflectivity and fluorescence spectroscopy experiments at the SOLEIL synchrotron enabled a detailed exploration of these interactions.
A well-known result of classical physics, that every student encounters at some point, is that interactions between charged surfaces in solution are generally well described by the Poisson-Boltzmann equation, known as the mean-field model. In this framework, two surfaces carrying charges of the same sign must repel each other. This approach explains many phenomena, from the behaviour of biopolymers to the stability of colloidal suspensions such as clay, certain paints, or latex.
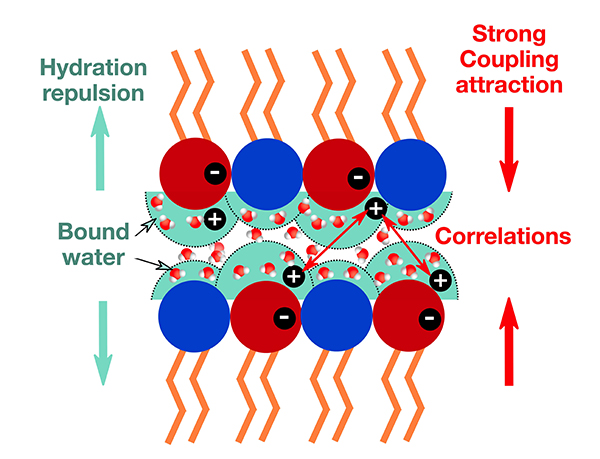
Surprisingly, under extreme conditions this description fails, and counterintuitive effects can emerge. For example, when highly charged surfaces are separated by only a few nanometres, interactions between ions can no longer be neglected, and the mean-field approximation is no longer valid. In particular, it has been observed that, under these conditions, surfaces with identical charges can attract each other! This phenomenon, already studied in the presence of multivalent ions (ions that have gained or lost more than one electron), plays a role in the cohesion of cement and in the condensation of actin, an essential protein of the cytoskeleton in living cells. To understand these effects, physicists use the ‘strong coupling model’, which relies on the strong correlations between ions confined in the thin layer of water separating the surfaces.
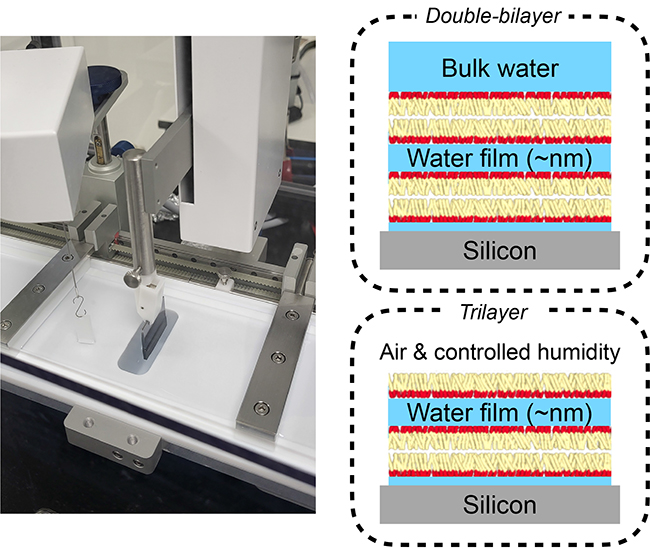
Several strong coupling models have been developed over the past thirty years, but simple and well-controlled experimental systems to test them remain rare. Supported lipid layers are one such model system. By stacking lipid monolayers one by one on a silicon surface, it is possible to create a “sandwich” in which a nanometre-thick layer of water is confined between two membranes whose composition and charge are precisely controlled.
The team at the Institut Charles Sadron and its collaborators have already explored this approach using two lipid bilayers (i.e. four monolayers) to study interactions between charged surfaces. The originality of the present work, carried out in collaboration with the Institut Laue-Langevin and the SIRIUS beamline, is the use of only three monolayers, the last one being in direct contact with air. This system offers two main advantages: it allows the ambient humidity to be controlled in order to exert an osmotic pressure on the stack by gradually drying it out, and it enables X-ray fluorescence spectroscopy measurements, which would not be feasible in the presence of a water volume above the layers.
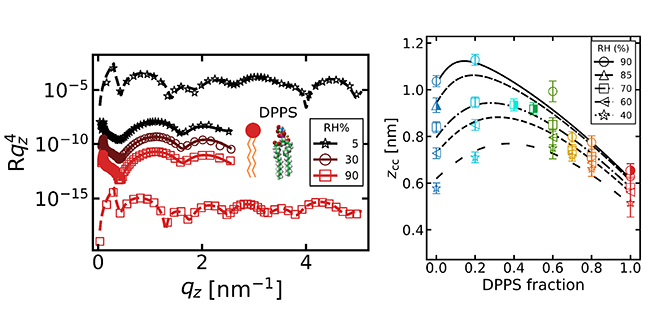
X-ray reflectivity experiments at SOLEIL and neutron experiments at ILL made it possible to monitor the water thickness as a function of humidity and surface charge. The results confirm those obtained with bilayers: lipid layers carrying the same charge do indeed attract each other, even in the presence of monovalent ions. The trilayer system enables a more systematic comparison with theoretical models, supported by numerical simulations carried out at the Institut Lumière Matière (ILM, Lyon). X-ray fluorescence spectroscopy was also used to rule out any contamination by divalent ions and to verify the exact proportion of charged lipids
The experimental results confirm the predictions of the strong coupling model, but they also highlight a crucial point: the role of interfacial water. Near hydrophilic and charged surfaces, water molecules do not behave as in the bulk liquid. They are strongly bound to the lipids, which modifies the local dielectric permittivity. Accounting for this effect is essential to achieve good agreement between theory and experiment. This work opens the way for a better understanding of interfacial water, a topic that shows similarities with observations made on graphene sheets in solution or in nanotubes with hydrophilic cores.