A vitamin D analog to fight advanced prostate cancer
Vitamin D is known for its anti-cancer activities, notably on prostate cancer progression, but it is of limited clinical use. Scientists from the Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) have demonstrated that a structure-based designed vitamin D analog has a larger therapeutic window1 than the natural vitamin D. They pinpointed the precise mechanism responsible for the anti-cancer activity of this analog. Moreover, they demonstrated that using this vitamin D analog in combination with docetaxel, the chemotherapeutic agent for advanced prostate cancer, overcomes chemoresistance to docetaxel.
The PROXIMA-2A beamline of SOLEIL contributed to these results.
Prostate cancer (PCa) is the third most common cause of cancer-related deaths in men. Androgen deprivation therapies are the standard care, but these therapies induce castration-resistant prostate cancer for which the treatment of choice is docetaxel. However, the efficacy of this chemotherapeutic agent is limited as most patients become chemoresistant.
The bioactive form of vitamin D (also known as 1,25D3) elicits its effects by inducing the transcriptional activities of its nuclear receptor (VDR) when binding to it. Hence, the binding between vitamin D and VDR has key regulator effects on calcium and phosphate homeostasis and also on various biological functions, such as cell growth, differentiation, anti-proliferation, apoptosis and adaptive/innate immune responses. Low circulating levels of vitamin D, and disrupted VDR transcriptional activities, have been shown to contribute to PCa progression. But vitamin D therapeutic doses promote calcium absorption leading to hypercalcemia, which is why a vitamin D analog with anti-inflammatory and/or antiproliferative activity, but without any procalcaemic activity is required. Numerous pre-clinical studies have reported a synergistic effect of docetaxel in combination with vitamin D, but the effects of vitamin D on chemoresistance in castration-resistant prostate cancer remain unknown. Researchers from the IGBMC have characterized a new vitamin D analog and explored its potency in combination to doxetaxel to restore docetaxel sensitivity in advanced prostate cancer.
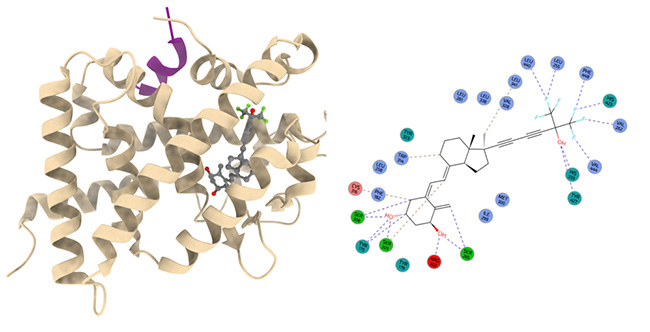
More than 4000 vitamin D analogs were synthetized, and some have demonstrated potent anti-inflammatory and/or antiproliferative activities in vitro, but few found clinical applications. IGBMC scientists designed the Xe4MeCF3 analog bearing a rigidified side-chain, a methyl moiety at carbon C17 and two trifluoromethyl groups (figure 1). They used the PROXIMA-2A beamline at SOLEIL to determine the three-dimensional structure of the VDR-analog complex and to study its binding mode. The structure of the complex at atomic resolution revealed additional interactions formed by the XE4MeCF3 compound, most notably the interactions with residues of the C-terminal helix caused by the fluorination of terminal methyl groups. The triple bonds that were introduced to lock the active side-chain conformation result in large gains in the entropic component of the binding reaction, and therefore, explain enhanced transcriptional activity of the receptor.
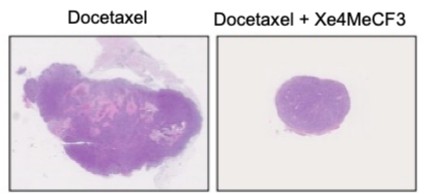
The activities of the analog were characterized indicating that Xe4MeCF3 is more potent than vitamin D to induce VDR transcriptional activities and has a wider therapeutic window. Importantly, Xe4MeCF3 in combination with docetaxel was shown to impair tumor growth by inducing cell apoptosis and reducing the aggressiveness of the remaining cells (figure 2).
The results gained from this study highlight key structure-function relationships for VDR activities and unravel the potency of VDR agonists to overcome docetaxel resistance of chemoresistant castration-resistant prostate cancer, opening new avenues for the clinical management of this refractory disorder with a high socio-economic impact.
¹ Therapeutic window: The dose range of a drug that provides safe and effective therapy with minimal adverse effects.