Tryptophan and gold nanoparticles: A winning duo for deep ultraviolet imaging of microbial cells
Researchers from the University of Belgrade successfully demonstrated the utility of tryptophan-functionalized gold nanoparticles as fluorescent probes for imaging of microbial cells. In the study, published in the Colloids and Surfaces B: Biointerfaces, the spatial distribution of hybrid nanoparticles has been determined within individual Escherichia coli cells using deep ultraviolet (DUV) fluorescence imaging, a high spatial resolution microscopy technique.
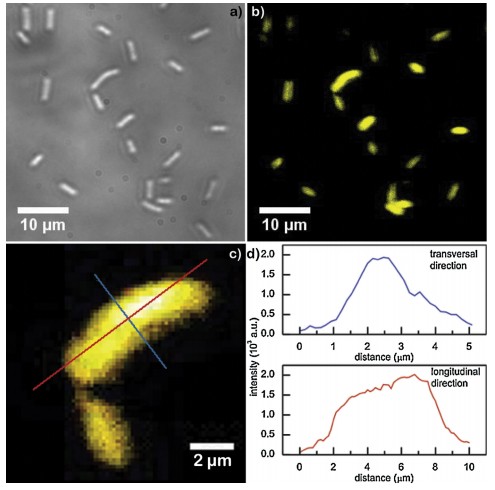
Could metal nanostructures soon be used in clinical applications? One step forward has recently been done this way. Researchers from the University of Belgrade achieved to follow, through deep ultraviolet (DUV) fluorescence imaging, the spatial distribution of tryptophan-functionalized gold nanoparticles within individual microbial cells. Tryptophan is a peculiar essential amino acid which absorbs and emits electromagnetic radiation in the ultraviolet domain. This property of the amino acid was attributed to the nanoparticles by attaching the biomolecules to the gold surface. Using the TELEMOS microscope of the DISCO beamline at the SOLEIL synchrotron facility, the researchers took a specific look to microbial cells’ fluorescence with and without the presence of the synthetized nanoparticles. Scientists observed a pronounced fluorescence in the UV domain (327-353 nm) from the cells incubated with the nanostructures, which they could clearly distinguish from the auto-fluorescence of the cells. Moreover, the signal intensity increased with an increase in the molar content of the amino acid used for surface functionalization. A high spatial resolution that can be achieved with DUV imaging technique also enabled researchers to determine the distribution of the gold nanoparticles within individual cells. The fluorescence signal intensity increased from the cell’s edge towards the interior, indicating that the hybrid nanostructures were mostly positioned around the central parts of E .coli cells.
In their study, the scientists also investigated the interaction between tryptophan and gold nanoparticles. Images obtained in electron microscopy (TEM and HRTEM) highlighted that gold nanoparticles tend to agglomerate in the presence of tryptophan. Their mean diameter increased from 8(+/-2) mm to 11 (+/-3mm). The UV-visible absorption spectra suggested that tryptophan induce a broadening of the surface plasmon resonance band of gold, together with a shift in its peak position towards higher wavelengths. These observations indicated the formation of tryptophan layers at the nanoparticle surface. Fourier Transform Infrared and X-ray photoelectron spectroscopies were used to determine the tryptophan groups involved in the interaction with gold surface. The observed changes in the tryptophan spectra implied that the carboxyl and indole groups are mostly involved in the interaction, with a slight contribution of benzene rings and primary amine.
Gold nanoparticles currently hold great promises in nanomedicine, because of their low toxicity, their biocompatibility, and their unique optical properties and stability in wide temperature ranges. However, due to their small size, they can be non-selectively absorbed. Coupling the nanoparticles with tryptophan enables detection of the nanoparticles in the cells by fast and non-invasive optical methods and it is a step toward better understanding of intracellular distribution of metallic nanostructures that could speed up their clinical application.