Transmission of nerve impulses - New structural data helps to understand the action of benzodiazepines on certain neurons
Benzodiazepines are among the most commonly prescribed psychotropic drugs in the world, used to treat anxiety, insomnia, convulsions, spasms, etc. These molecules act by binding to specific sites at the end of certain neurons and modulating the transmission of nerve impulses. To complete the information on the mechanisms controlling this transmission, a group of European researchers determined the 3D structure of a neuronal receptor when bound to different psychoactive molecules. These results, published in PNAS, are largely based on diffraction data obtained on the PROXIMA 1 beamline.
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the central nervous system of humans and other animals. This molecule, secreted by certain neurons, is involved in the regulation of neuronal activity. It belongs to the family of neuromodulators, with dopamine, serotonin and glutamate as other examples. The inhibitory effect of GABA, for example, counteracts the excitatory effects of glutamate.
GABA’s action is initiated by binding to receptors present at the ends of neurons. There are two families of GABA receptors: GABAA and GABAB.
GABAA receptors are members of the so-called pentameric ligand-gated ion channel (pLGIC) family involved in inhibitory neurotransmission. This family also includes glycine receptors, nicotinic acetylcholine receptors and serotonin 5-HT3 receptors. In this type of channel, the 5 sub-units are arranged around a central pore, and when the ligand (GABA, glycine, etc.) binds to its binding site, the channel opens, letting anions through the pore. In the case of GABAA receptors, GABA binding opens the channel, leading to the influx of Chloride (Cl-) ions into the neuron, resulting in hyperpolarization of the neuron and its inhibition: the nerve impulse is no longer transmitted.
How GABAA receptors work
These GABAA receptors therefore have binding sites for GABA, but also for benzodiazepines1 and other regulatory molecules, with binding resulting in a change of conformation of the different subunits of the receptor. This conformational change alters the GABA/receptor binding site which, in the case of benzodiazepines, increases GABA/receptor affinity. The increased frequency and duration of the Cl-channel opening leads to greater nerve impulse inhibition.
Despite extensive studies on GABAA receptors, and in particular the GABA and benzodiazepine binding sites, X-ray structural data are still scarce on GABAA receptors in eukaryotic cells. On the other hand, the 3D structures of several channels of the 'pLGIC' family to which GABAA receptors belong are known: two bacterial channels, ELIC and GLIC and the glutamate-gated chloride channel (GluCl) of a eukaryote (nematode, C. elegans). These data revealed the molecular architecture of open and closed states of the receptor, and the determinants for ligand binding.
What ELIC and GABAA have in common
The authors of the findings published in PNAS investigated whether these channels with the already known 3D structures interacted with the same molecules as those that bind to the GABAA receptor in eukaryotes. Using high-throughput electrophysiological screening of a library of compounds (amino acids, intermediates of photosynthesis, neurotransmitters, agonists and modulators of ion channels sensitive to ligands), they discovered that the ELIC ion channel was activated by GABA and could be modulated with benzodiazepines, with similar effects to eukaryotic GABAA receptors. Given these "behavior" similarities, the researchers used ELIC for new structural studies.
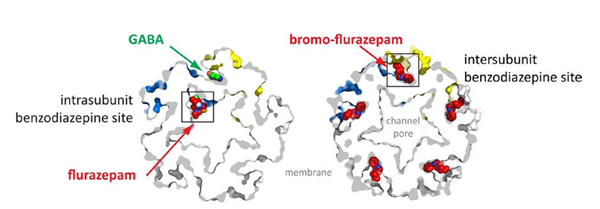
The X-ray structure of ELIC in a complex with GABA and benzodiazepines was obtained from X-ray data obtained on PROXIMA 1 and at SLS. It revealed that GABA binds to the extracellular domain at the sub-unit interface, where it remains trapped by aromatic residues in the manner of the agonist interactions observed in acetylcholine binding proteins and the GluCl channel.
Researchers thus observed that benzodiazepines can occupy two different ELIC binding sites, depending on their concentration. The intra-subunit binding site is located in a pocket localized opposite the GABA binding site, facing the channel vestibule, which potentially corresponds to a low affinity benzodiazepine binding site for GABAA receptors. Located between the two subunits, the site is occupied by an analogue of flurazepam1 or by zopiclone1 (data with zopiclone were obtained at SLS) and resembles the high affinity binding site of GABAA receptors.
These results provide a structural view of how GABA and modulator molecules are recognized on different binding sites of pLGIC-type channels, providing new elements to improve our understanding of the activation and modulation in this family of ion channels.
1- Benzodiazepines are a class of chemical compounds used as psychotropic drugs targeting neurotransmitters of the central nervous system by increasing their inhibitory action on neurons. Valium is historically the first drug of this family to have been used. Flurazepam is also a benzodiazepine.
Zopiclone is a hypnotic drug pharmacologically close to but not chemically one of the benzodiazepines.