Three water molecules are enough to stabilize a dipeptide
Experiments carried out at DESIRS beamline show that nanosolvation of a fragile peptide dimer by only three water molecules, isolated in the gas phase, has a dramatic impact on its stability. A drastic reduction of the fragmentation abundance, upon VUV irradiation, of the hydrated protonated dimer precursor was observed by mass spectrometry. This effect is well reproduced by calculations showing that hydration indeed stabilizes the dimer structure. These results are published in Angewandte Chemie.
The structure and functionality of biomolecules are intrinsically linked with their aqueous environment. There has been a long-standing effort to understand the effect of the surrounding water solvation shell on the conformation of biomolecules and how it influences both their functional properties and susceptibility to external factors (such as ionizing radiation). In particular, the solvent has a key role in self-assembling processes and in the formation and stabilization of macromolecular complexes.
The limit of solvation: how many water molecules are needed to produce an effect?
The need to gain a deeper understanding of the effect of water solvation on the protein structure has led to an intensive investigation of gradual solvation of biomolecules – the limit of the so called microsolvation (or nanosolvation), referring to only a small and well-defined number of attached water molecules, has been conceived. However, the experimental investigation of nanosolvated species under well-defined conditions is challenging. The present study brings a comparative experimental and theoretical study on the bare protonated leucine–enkephalin peptide dimer ion and on the same system solvated with three water molecules, showing already a clear nanosolvation effect. The Leu-Enk dimer is used here as a model system for peptide–peptide interactions, which is pertinent not only for non-covalent complex formation but also for the acquisition of secondary and ternary structures of proteins.
Measuring VUV photon-induced fragmentation of a bare and a solvated peptide dimer
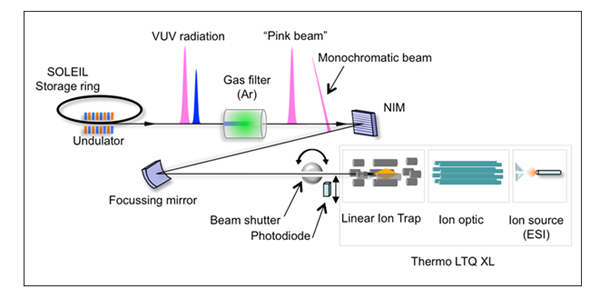
By using a recently developed experimental setup (ref. 1), in which an ion trap mass spectrometer equipped with electrospray sources is coupled to the DESIRS VUV beamline, it has been possible to isolate in the gas phase a bare and a solvated with 3 water molecules protonated dimer of Leu-Enk, and to measure the photon-induced fragmentation intensity at different photon energies.
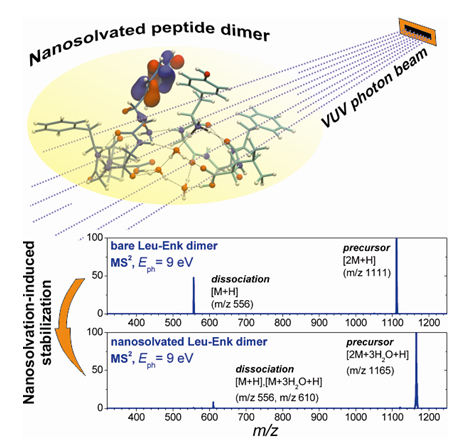
The results showed a drastic suppression of both the hydrated precursor dissociation into monomers (figure 2) and the peptide backbone cleavage, thus clearly demonstrating a dramatic stabilization of the system due to the addition of only a few water molecules.
Furthermore, the solvation-induced stability was substantiated by detecting the doubly charged ion produced upon photoionization of the hydrated protonated species, which can be preserved in the gas phase, thus also allowing a precise determination of the ionization threshold. The apparent increased stability toward VUV irradiation of the hydrated complex with respect to the bare species is striking and questions the energy dissipation process, since the nanosolvation produces a frustrated dissociation in the dimer even for irradiation at energies below the ionization threshold.
Calculations confirm that hydration stabilizes the dimer structure
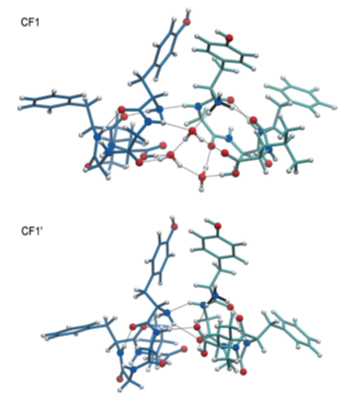
Theoretical study, employing molecular dynamics and density functional theory (DFT) calculations, of the structure of the nanosolvated peptide dimer is consistent with the experimental findings. The calculations show that hydration with only 3 water molecules does not affect significantly the 3D structure of the dimer (see figure 3), but rather stabilizes it by about 1.5 eV. Actually, the dimer is formed via non-covalent bonds (H-bonds) both in the bare and solvated cases. However, comparison of the bare and hydrated complexes reveals that the binding is strongly enhanced upon nanosolvation, which is due to the flexibility of H2O to adjust its bridging position and orientation with respect to the other molecules, thus increasing the effective number of hydrogen bonds.
The present results are important to consider when stability of protein non-covalent complexes and protein structure is assessed for isolated non-hydrated ions, by mass spectrometry for example. Moreover, the phenomenologically shielding effect of microsolvation observed here is of interest in the field of radiation damage and could help in the future to have a better understanding of these processes at the molecular level.
Référence :
1 - Milosavljević A R, Nicolas C, Gil J-F, Canon F, Réfrégiers M, Nahon L & Giuliani A
VUV synchrotron radiation: a new activation technique for tandem mass spectrometry
Journal of synchrotron radiation, 2012, 19 174–8