Shaken, not stirred - a spy story at the atomic level
Xenon is a fascinating but rare element. For an atomic physicist it shows itself as a horn of plenty: due to its electronic structure it provides a lot of transitions with complex electron correlation effects to study (Xe atom has 54 electrons) and requires accurate modeling of relativistic effects. As a rare gas it is a lonely rider: in normal conditions it exists as individual gas-phase atoms without a need to interact with others. In low enough temperatures it is nevertheless possible to get a bunch of Xe atoms to stick together, to form a cluster.
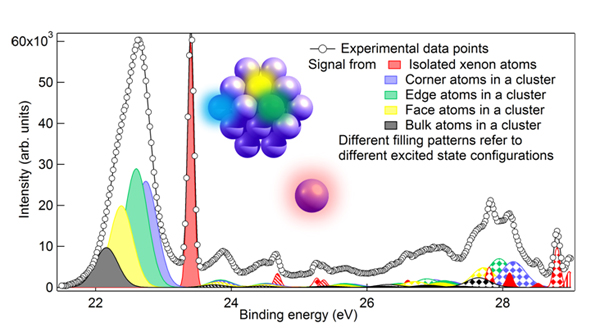
In a recent study at the PLEIADES beamline, researchers have used extremely small agents, electrons, to investigate the structure of small xenon clusters. The idea is to “shake” the clusters by soft x-rays causing a process which both ionizes and excites a Xe atom in a cluster. The excited electron serves as a spy: it can enter to the space of the neighboring Xe atoms (overlap of the wavefunctions of the electron and the neighboring atoms) and, through so-called exchange interaction, lurks its surroundings. The electrons can be excited only to specific orbitals having certain properties governed by laws of quantum mechanics, such as a radial distribution and an angular momentum (favored places to exist around the atom). Due to these properties the electrons excited to different orbitals feel the surrounding atoms differently. On the other hand, in a small cluster not all Xe atoms are equal: some of the atoms are on the surface of the cluster, and they have less neighboring atoms than the atoms that are inside the cluster. Thus the “intelligence information” obtained by the agent electron also depends on where in the cluster the excited atom is.
The information received from the different type of spies (electrons excited to s, p, or d type of orbitals) is recorded as a photoelectron spectrum: the kinetic energy (velocity) of the ionized electron depends on the final state of the whole cluster. By analyzing these kinetic energies the researchers were able to deduce that most likely the small Xe clusters have an icosahedral structure: they saw signal from four different locations of the cluster: from atoms completely surrounded by other atoms (in the bulk) and from atoms located at three different positions on the surface of the cluster with different amount of neighbors.
Next step: When shaking, beware of the spills!
In this study it was assumed that the ionization-excitation process shakes the cluster gently, and the recorded electrons represent the geometry of cluster more or less in its ground state geometry. However, it is known that these kind of excited states are not lasting for a long time: in some cases the agent electron returns back to the base camp in femtoseconds (10-15 s). While the excited system decays to the ionic ground state, energy is released. The fact that there is excess energy in the cluster can lead to a second ionization process. What is remarkable is that this “extra ionization” can take place only if the Xe atom is embedded in a cluster; the process is not possible in an isolated atom. Thus it can for example happen that suddenly there are two ionized Xe atoms in a cluster, which start to repel each other, and as a result the cluster breaks down. Very recently (June 2013) the researchers continued the studies of clusters by also analyzing, in addition to the electrons, the ions that are left after the fragmentation of the cluster. The “spilt” ions will give additional information of the fundamental processes taking place after the soft x-ray shaking and the role of the fellow atoms in these weakly bonded systems.