RNA splicing - the lariat conformation captured at PROXIMA-1!
Group II introns are large catalytic RNAs and mobile genetic elements of bacterial origin. They are considered to be the ancestors of nuclear introns in eukaryotes and their splicing machinery, the spliceosome. In both splicing systems the intron is released in a ‘lariat’ conformation carrying a specific 2’-5’ phosphodiester bond. X-ray diffraction data collected at PROXIMA-1beamline by scientists from the Institute for Integrative Biology of the Cell (I2BC) allowed to solve two crystal structures of a group II intron lariat primed for reverse splicing. These structures illuminate the raisons for the extreme conservation of the lariat conformation during the evolutionary path from group II to spliceosomal splicing. This work was published in Science, December 2, 2016.
Group II introns are catalytic RNAs (ribozymes) able to splice from precursor RNA molecules in the absence of proteins. These large ribozymes, whose secondary structure is organized into six domains (I to VI), are abundant in bacteria and in the bacterial-derived organelles (mitochondria and chloroplasts) of some higher organisms. Bacterial group II introns also encode a reverse transcriptase (RT) enzyme and, in association with their RT, behave as mobile genetic elements that colonize genomes through retrotransposition.
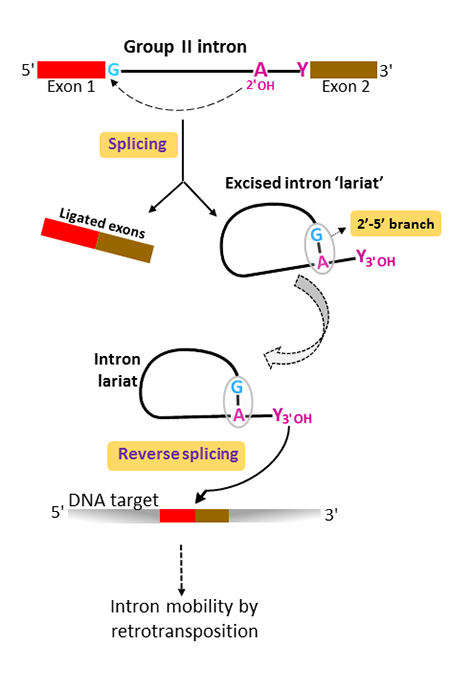
Although group II introns originated in bacterial world, they are believed to have played a determinant part in eukaryotic evolution as the ancestors of nuclear premessenger introns and their splicing machinery (the spliceosome). Both group II and nuclear pre-mRNA splicing proceed via two sequential phosphoryl transfer reactions and result in the ligation of the flanking 5’ and 3’ exons and the release of the intron as a branched molecule called ‘lariat’ (figure 1). The branched conformation results from a specific 2’-5’ phosphodiester bond linking a highly conserved intron adenosine to the first intron nucleotide, most often a guanosine. In group II introns, the branchpoint adenosine lies in the small helical domain VI, at the 3’ end of the molecule. Excised group II intron lariats initiate retrotransposition by catalyzing their own insertion into a DNA target, a mechanism known as ‘reverse splicing’.
The structural basis for the evolutionary success of the lariat conformation, which had been left unexplained for thirty years, is now brought to light by Maria Costa and co-workers at the Institute for Integrative Biology of the Cell (I2BC) who crystallized a group II intron lariat either alone or bound to an unreactive analog of the 5’ exon.
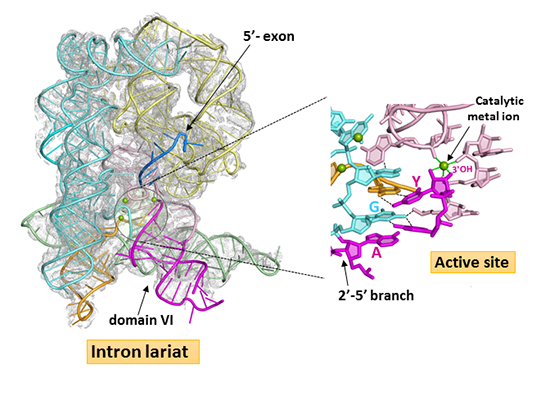
X-ray diffraction data collected on the PROXIMA1 beamline allow to solve the structures of the unbound and 5’-exon-bound lariat forms at 3.4 and 3.5 Å resolution, respectively. This work, recently published in Science, reveals for the first time that the 2’-5’ branch is essential in organizing the intron active site for efficient and faithful ligation of the flanking exons during the last stage of splicing. In the resulting architecture the conserved intron boundaries are juxtaposed and the last intron nucleotide positioned in the catalytic center. Furthermore, the 2’-5’ branch structure is seen to lock the active site of the excised lariat into a configuration poised for catalysis of the reverse-splicing reaction, the first step in retrotransposition (figure 2).
X-ray diffraction data from ytterbium-derivative crystals were collected on the PROXIMA1 beamline at the Yb-edge and used to generate an Yb3+ anomalous difference map which revealed two strong binding sites in the reaction center. Ytterbium mimics magnesium binding to RNA and the magnesium binding sites inferred from these data are consistent with the reverse-splicing reaction proceeding by the ‘two-metal ion’ mechanism, the general mechanism used for catalysis of phosphoryl transfer reactions.
Interestingly, these new crystal structures also reveal that assembly of the functional active site is under tight conformational control: a rearrangement of the base-pairing pattern of domain VI is required for stable docking of the branch structure into the active site and binding of the 5’-exon drives an induced fit that contributes to coordination of one of the two catalytic metal ions.
Finally, the present work reinforces the evolutionary relationship between group II and nuclear premessenger splicing in eukaryotes by suggesting new functional homologies between nucleotides conserved in both systems.
To date, a few group II introns have been developed into gene targeting vectors with programmable DNA target specificity; such vectors are already widely used for genetic manipulation of bacteria. The structural data stemming from the present work will help the rational design of additional gene targeting vectors with novel properties that, among other applications, could be used for genomic engineering of eukaryotes.