Prussian blue conservation: what if the substrate were more important that the pigment?
A study conducted at the SOLEIL synchrotron facility by researchers from the Bern University of Applied Science, the Conservation Research Center and from SOLEIL on Prussian blue fading process in heritage objects showed a redox mechanism regulated by the pigment’s substrate.
Prussian blue is a ferric ferrocyanide compound fortuitously discovered at the beginning of the 18th century by a color-maker and which has lived several life since. First used in paintings, it then served as a marking layer in boilermaking and for the prevention of some radioactive contamination (such as Cesium 137). It is now one of the major compounds in materials sciences, especially appreciated for its magnetic, electrochemical and optical properties tuned by external stimuli. This pigment could then lead to some promising applications, such as battery fabrication, memory device, and biosensors.
Despite this wide use, Prussian blue has remained elusive on several aspects. Its fading when submitted to light exposure, which depends on the object, still has to be clarified. Scientists assume that this process is related to the substrate, the environment, the pigment’s structure, and impurities.
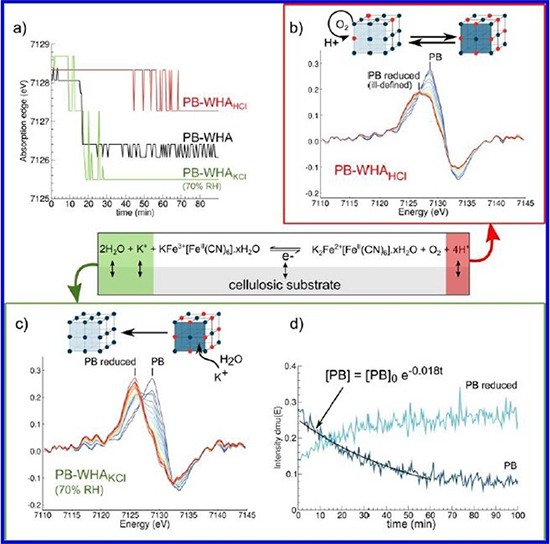
A team of researchers from the Bern University of Applied Science, the Conservation Research Center(CNRS-MNHN-MCC), and SOLEIL came on the ODE beamline to study the substrate’s impact on the fading phenomenon. Prussian blue samples deposited on several paper matrixes have been irradiated by a 1.1 x 1011 photons/s flux X-ray beam and studied by X-ray Absorption Spectroscopy, an appropriate technique to determine both the oxidation state and the environment of iron atoms, but also their potential changes during the pigment’s fading. But here is the thing: the Prussian blue is equally sensitive to visible light than to X-ray radiations. The team then first characterized those X-ray induced damages and showed a two-step phenomenon. The first one consists in a pigment’s reduction similar to the one observed after a visible light exposure, leading to a shift of the absorption edge toward lower energy from 2 eV after 16 minutes of exposure. When the reduction is complete, the second step consists in the Prussian blue irreversible degradation in iron (III) oxyhydroxyde.
(a) Evolution of the absorption edges with X-ray exposure time. Evolution of the XANES derivatives for
(b) PB:WHHKCI and
(c) PB:WHAKCI at 70% RH. Spectra are colored according to a rainbow color scheme, from t=0 (blue) to t=120 min (red).
(d) Evolution of the height of the derivatives at 7128.5 eV (dark-blue curve) and at 7125.5 eV (light-blue curve) in the system PB:WHAKCI (70% RH). Center: General redox equation for the Prussian blue-paper system.
The team led by Claire Gervais especially took interest in the first step of photoreduction: X-rays permit a pigment’s reduction way faster than visible light, and it is now possible to instantly follow entire photoreduction kinetics and to study the substrate’s impact in this redox process. The researches particularly studied flux of both cations and protons associated to the iron reduction by comparing kinetic reduction under X-ray of two modified Prussian blue systems: one with an acidified paper matrix, the other with a paper matric was artificially loaded in potassium and kept in a humid environment. Compared to the reference sample, the reduction is less important (1 eV) and occurs later (50 minutes) in the acidified sample. On the contrary, the reduction is more important (3 eV) and occurs sooner (10 minutes) in the sample loaded in potassium. Those two observations allowed the scientists to sharpen the redox mechanism responsible of the Prussian blue fading. Indeed, for electroneutrality purposes, the iron (II) reduction must be compensated by an input of cations within the Prussian blue structure. The matrix paper loaded in potassium, combined with a humid environment, creates a solvated cations reservoir, able to migrate within the pigment and then promote the Prussian blue reduction reaction. As for the acidified paper matric, the protons brought and the ambient oxygen promote the re-oxidation of the reduced Prussian blue, then acting in opposition to X-rays. This opposition leads to a ‘frustrated’ system, with lower reduction kinetics, and to the creation of disorders within the structure, resulting in a heterogeneous distribution of different oxidation states within the Prussian blue lattice.
By stressing the key role played by substrate/pigment exchanges during Prussian blue fading, those results pave the way to preventive strategies for art containing Prussian blue: in order to mitigate fading caused by either visible light or X-ray during the sample analysis, it is necessary to focus at least equally, if not more, on the substrate and its photosensitivity that on the pigment itself.