Proteins easy to peel... of electrons!
Removing electrons from a material requires energy that can be provided in various forms. When using light as the energy source, it is by the photoelectric effect that electrons are ejected from neutral matter (atoms, molecules), via the so-called photoionization process. When the initial targets are negatively charged ions, this is referred to as photodetachment, a process that requires little energy (less than a few eV) due to the lower Coulombic attraction than in a neutral species, experienced by the outgoing electron.
In the past, this process has been widely studied in the visible and near UV, using lasers which energy is high enough to induce photodetachment. But very little research has focused on the highest-energy VUV field (far UV). How would initially negatively-charged matter (called anions) behave irradiated with VUV radiation?
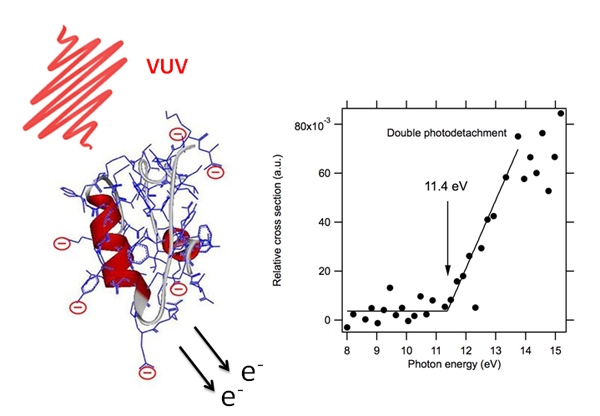
Through the development at SOLEIL of an innovative coupling between VUV synchrotron radiation and mass spectrometry, researchers at Lyon Claude Bernard University-CNRS and SOLEIL, have shown that electron photodetachment was the main mechanism observed after irradiation with VUV photons (6 to 20 eV) of model proteins (such as insulin), which carry multiple negative charges (polyanions, see Figure 1), regardless of the initial anion’s state of charge. If the irradiation time is long (hundreds of milliseconds), the protein can even sequentially eject several electrons (there is then a true peeling away of electrons from the protein!).
Even more spectacular, this study showed for the first time a direct double detachment of valence electrons in these proteins. Double sequential photodetachment (mainly by Auger relaxation) from inner valence shells had already been reported (using ion beams and synchrotron radiation techniques) of atoms and small molecules. Here, the threshold for direct ejection of the two electrons is measured at 11.4 eV (see Figure 1), much lower than those measured for inner shell double photodetachment. This direct double photodetachment, a signature of strong electronic correlations in the protein, appears to be a relaxation mechanism that leads to oxidized anions that keep the original structure of the protein, a feature of great importance in radiobiology. Indeed, a striking observation on the recorded mass spectra is the stability of the oxidized protein (with very little fragmentation) over the time scale of the experiment, even in the case of multiple photodetachments.
This discovery opens very rich alleys of basic research. In particular, the energy correlation between the two electrons emitted and the exact electronic structure of the ion produced should be studied in detail in the future, in order to understand both the dynamics of the process and its implications in terms of structure. This means understanding how the energy deposited within the system is flows among the different electronic (energy emitted by the two photoelectrons) and nuclear degrees of freedom which could, or could not, lead to the fragmentation of the molecule.
Reference :
Antoine, R. & Dugourd, P. Phys. Chem. Chem. Phys. (2011) 13,16494.