Probing subtle conformational changes in chiral gas phase molecules
Chirality plays a fundamental role in molecular recognition processes, as those involved in metabolism. Molecular flexibility is also crucial in molecular recognition, allowing the interacting molecules to adjust their conformations hence optimize their interactions. Methods probing simultaneously chirality and molecular conformation are therefore crucially needed. By playing on certain experimental conditions that affect conformations, a collaborative consortium based upon a team of ISMO (CNRS Univ. Paris-Saclay) and the DESIRS beamline team demonstrated the exquisite sensitivity of photoelectron circular dichroism (PECD) to both chirality and subtle conformational changes.
Chiroptical spectroscopy techniques, i.e. spectroscopy sensitive to chirality such as circular dichroism (CD), are widely used to characterize the structure of biomolecules. CD measures the difference in the capacity of a chiral molecule to absorb right and left by Circularly Polarized Light (CPL). However, the lack of sensitivity of CD, makes its application to the gas phase -i.e. on very diluted samples- extremely challenging.
In contrast, Photoelectron Circular Dichroism (PECD) is ideally suited for studying gas-phase molecules. When chiral molecules are ionized by CPL, the direction of the electrons which are then photoejected depends on the polarization (right/left) of the CPL: there is a forward-backward asymmetry in the photoelectron angular distribution with respect to the ionizing light propagation axis. This forward-backward asymmetry (the PECD) is very large, reaching up to tens of %, reverses when the enantiomer is changed, i.e., from a left to a right form, and is zero for a non-chiral system.
PECD depends on the orbital from which the photoelectron is ejected. PECD also is a delicate probe of the chiral molecular potential (i.e., the structure of the molecule), and is theoretically predicted to be highly sensitive to conformations, which is usually verified experimentally only indirectly.
In the present work, the CPL from the DESIRS beamline was coupled to an electron/ion double imaging coincidence spectrometer. The researchers have been studying the case of ring inversion in 1-Indanol (see fig.1), a floppy chiral species. When the carrier gas used to form the 1-Indanol molecular beam is Ar, only the most stable conformer, 1eq, is formed, while with He a mixture of conformers is produced with 2ax and 1eq in a 0.25:1 proportion. This unique property offers the opportunity of directly observing and controlling the PECD behavior of a flexible molecule.
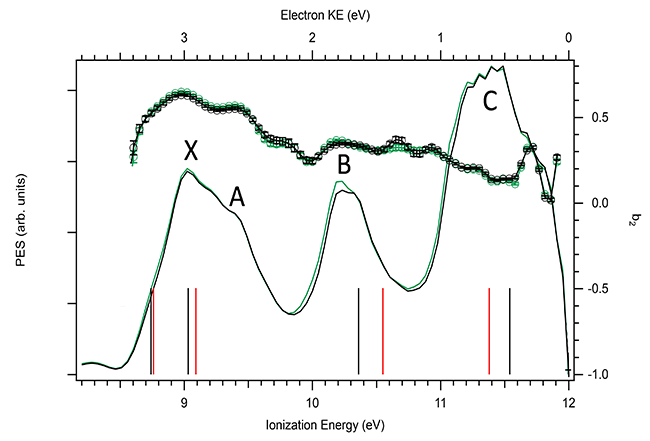
Let’s first observe the conformational sensitivity of the two other (and non-chiral) observables in molecular photoionization: the cross sections (s) as given by the Photoelectron Spectrum (PES) show the ionization probability from the various electronic orbitals and b2 (=- ½ b, the usual anisotropy parameter). The variation of these two parameters for the first 4 orbitals (labelled X to C) is shown in Fig.1, for both Ar and He carrier gas. Clearly there are no differences in terms of carrier gas and therefore in terms of conformations: s and b2 are poorly conformer-sensitive and as such cannot provide much structural information.
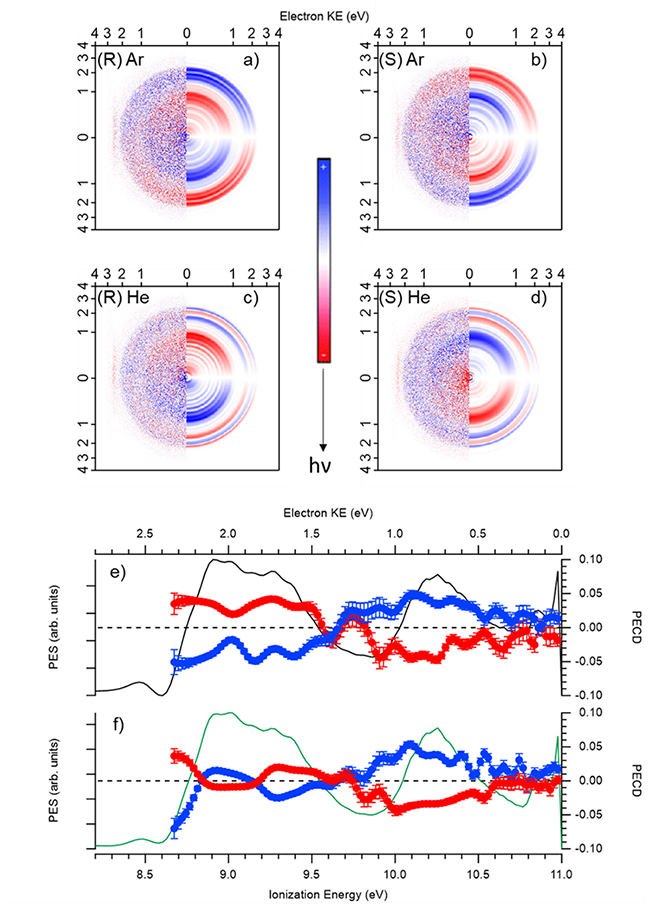
At the contrary, PECD appears as very strongly conformer distribution dependent. This can already be observed on the difference electron images (left-CPL – right-CPL), in which for both enantiomers, clear differences can be seen especially in the outer rings of the images shown in Fig.3 (3a and 3b vs 3c and 3d). More quantitively, the PECD curve, besides showing a quasi-perfect expected mirroring between the two enantiomers, strikingly exhibits a massive difference and even a sign inversion in the region of the highest occupied orbital (HOMO) ionization (band X) depending on the carrier gas, i.e., on the conformer distribution (Fig. 3e vs 3f). This shows that the b1 parameters associated to the 1eq and 2ax conformers are opposite in sign for the HOMO, with a much larger PECD magnitude for the 2eq conformer.
The present results prove the exquisite conformational sensitivity of PECD, in a case where the PES as well as the b parameter are genuinely superimposable. Such a situation is quite common, so that the outcome of this study may have strong consequences for the ongoing development of laser-based PECD techniques in an analytical context, with possible conformer-selectivity in the future.
GLOSSARY
Chirality / enantiomer: An object is “chiral” if it exists as two non-superimposable forms which are mirror image of each other. This is the case of hands (or feet!), propellers, but also of many molecules (for example, the ones with an asymmetric carbon linked to 4 different chemical groups) – the two forms are called left or right enantiomer.
Conformers: many molecules and in particular biomolecules, are floppy, existing as several conformers, obtained by rotations of chemical groups around a chemical bond and by deformation (puckering) of a ring.
Circularly Polarized Light (CPL): Polarization is a geometric property of light, which describes the motion of the electric field associated with the light wave as it propagates. In the case of circularly polarized light, the electric field describes a helix (like a corkscrew) which can be clockwise or counterclockwise, defining then CPL with left or right helicity. Note that CPL is a chiral object.
Photoemission / photoionization: action of light tearing off an electron from matter. It corresponds to the photoelectric effect: the energy of the incident photon (hn) is shared between extraction work of the electron (binding energy) and the kinetic energy of the emitted photoelectron (Ek). Photoelectron spectroscopy (PES) measures the distribution of Ek energies for a given incident photon energy, allowing the determination of the (binding) energy levels of the electrons in the molecule (which corresponds to the molecular orbitals energies). One can also measure in which directions the photoelectrons are ejected with respect to a given axis: i.e angular distributions. This distribution (characterized by the b parameter) does not have the same properties in all directions, in other words: is not isotropic (b ≠ 0) because the molecule "remembers" the polarization of the incident light.
PhotoElectron Circular Dichroism (PECD): As CPL is a chiral object, it will induce enantio-specific processes upon interaction with a given enantiomer of a chiral species: these are circular dichroisms, a chiral recognition between chiral objects, such as a left glove "recognizes" a left hand. In the case of photoemission of a given enantiomer of a chiral molecule by an CPL of a given helicity (left or right), the PECD manifests itself by a forward / backward asymmetry with respect to the light propagation axis, which is not found for non-chiral species. This asymmetry is reversed when the enantiomer or light helicity is swapped. The specificity of PECD, as compared to other types of circular dichroisms, lies in its intensity: from a few % to a few tens of %. It is also very sensitive to molecular structures such as isomers (molecules with the same atomic composition, but a different arrangement of these atoms), or conformers.