Operando study of catalytically active interfacial metal-oxide nanostructure
Bimetallic materials have opened a new pathway that could control the electronic structure and chemical bonding in catalysts, resulting in superior catalytic performance. Despite considerable focus on various catalytic reaction studies, there are still many questions unanswered for underlying causes of improved performance. The variations of the structure, chemical composition, and oxidation state of bimetallic materials under reaction conditions complicate the understanding of the origin of greater catalytic activity of bimetallic materials.
Combining ambient pressure x-ray photoelectron spectroscopy at TEMPO beamline of SOLEIL and ambient pressure scanning tunneling microscopy in Japan, Researchers from Korea (Institute of Basic Science, Korean Advanced Science and Technology, Gwangju Institute of Science and Technology), Japan (Keio University, Institute of Materials Structure Science, PF-KEK), and France (Sorbonne University) showed that the formation of metal-oxide interfaces is the key factor that is responsible for the catalytic synergistic effect in bimetal catalysts.
Platinum-nickel (Pt-Ni) bimetallic catalysts has been well known for its superb catalytic activity for electrochemical and heterogeneous catalytic reactions such as oxygen reduction reaction (ORR) of hydrogen fuel cell and CO oxidation. Recent studies revealed the presence of metal-oxide interfacial sites formed by surface segregation of bimetallic nanoparticles were closely related to the increased catalytic activity. Nonetheless, the physical/chemical nature and fundamental role of oxide–metal interfaces still remain elusive because of a lack of definitive evidence.
To answer to this intriguing question, international research teams from Korea (Institute of Basic Science, Korean Advanced Science and Technology, Gwangju Institute of Science and Technology), Japan (Keio University, Institute of Materials Structure Science, PF-KEK), France (Sorbonne University), and SOLEIL synchrotron performed a collaborative research. Combining ambient pressure x-ray photoelectron spectroscopy (AP-XPS) at TEMPO beamline of SOLEIL and ambient pressure scanning tunneling microscopy (AP-STM) in Japan, the team led the collaborative research on the fundamental understanding of reaction mechanism on bimetal catalysts.
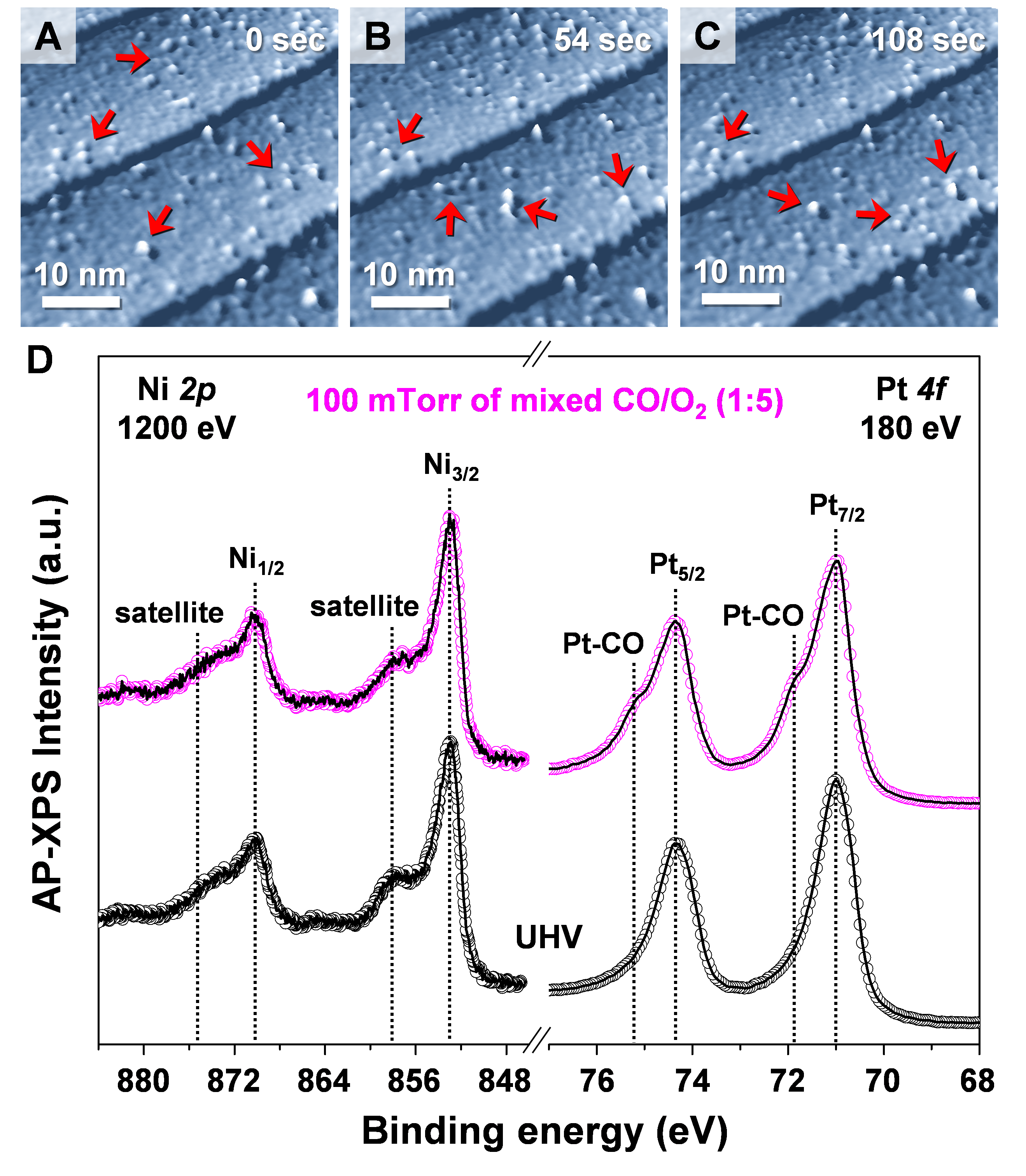
The team carried out in-situ observation of structural modulation and chemical variations on Pt-Ni bimetallic catalysts under CO oxidation conditions and displayed that Pt-Ni bimetallic catalysts exhibiting various structures depending on the reaction conditions. Under ultrahigh vacuum conditions, the surface exhibited a Pt-skin layer on the Pt-Ni alloyed surface, and selective Ni segregation followed by the formation of Ni oxide clusters under oxygen gas, and finally the coexistence of Ni oxide clusters on the Pt-skin during the CO oxidation. (Fig. 1) Especially, during CO oxidation condition, the enhancement of CO oxidation occurs with the formation of interfacial Pt-NiO1-x nanostructure on the surface.
The research team found that the formation of interfacial Pt-Ni oxide nanostructures is responsible for a highly efficient step in the CO oxidation reaction. This is the first visualization of bimetallic surfaces under reaction conditions, and a demonstration of the significance of metal-oxide interfaces in the heterogeneous catalysis. Furthermore, the team calculated thermodynamically favored reaction pathway for Ni oxides on Pt-Ni surface using density functional theory. In conclusion, the research teams confimed that the interfacial metal–metal oxide nanostructures provide thermodynamically efficient reaction route by lowering the heat of reaction on surface, which results in its superb catalytic performance.