Nitric acid on snow flakes…
Nitric acid HNO3 is an important player in a plethora of environmental heterogeneous processes involving atmospheric aerosols, icy particulates and snow. Whether nitric acid adsorbs in its molecular form HNO3 or dissociated form NO3- (nitrate) on these environmental surfaces is a key question in the formation, stability, and reactivity of nitrates in urban particulate matter, the formation and lifetime of cirrus clouds, and the budget of atmospheric nitrogen oxides over snow-covered regions.
On the TEMPO beamline, French and Canadian teams have studied by NEXAFS spectroscopy the conversion of nitric acide into nitrate on water ice, as function of the ice temperature.
The atmospheric nitrites and nitrates (NOx) released from the UV-visible photolysis of molecular HNO3 trapped in the snowpack would be 400 % higher than that of NO3-, hence the existence of a stabilized molecular form of HNO3 in water ice would have a huge impact on the NOx fluxes over the Earth’ cryosphere. So far, no satisfactory answer to whether HNO3 is dissociated in/on water ice exists, which has motivated the characterization of the chemical form of HNO3 adsorbed on ice, using NEXAFS spectroscopy on TEMPO.
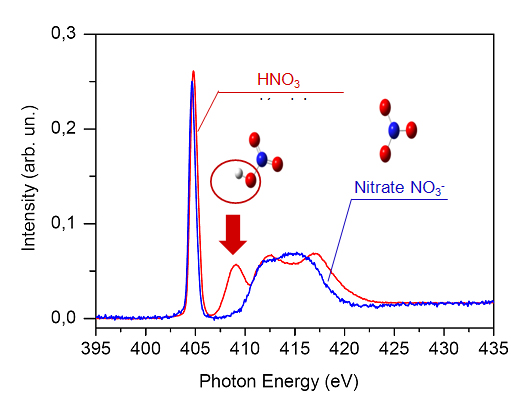
Because NEXAFS spectroscopy probes the electronic structure, and because HNO3 and NO3- have different ones, their NEXAFS spectra might be different too. This is indeed the case (figure 1), especially around 409 eV where the molecular HNO3 spectrum (red) displays a strong resonance (arrow) specific to the hydroxyl group OH, then absent in the NO3- one (blue). Therefore, investigating the conversion of nitric acid HNO3 into nitrate NO3- on water ice is particularly convenient given that spectral feature at 409 eV.
A hundred monolayers for a snowflake
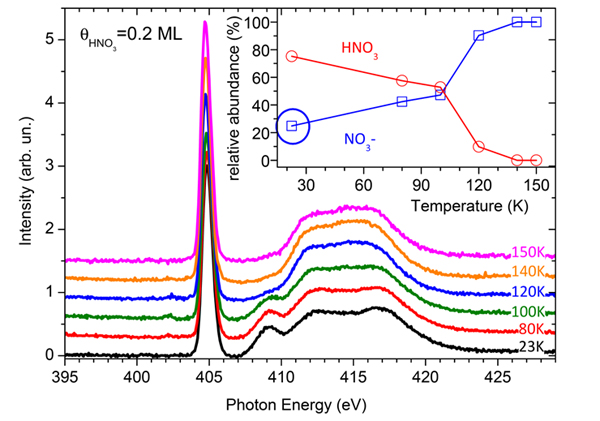
The scientists have studied the extent of ionization of HNO3 on the surface of a thin water ice film (100 monolayers thick), as a model of snowflake in interaction with nitric acid. Figure 2 displays the evolution of the N K-edge signal for 0.2 monolayer of HNO3 adsorbed on ice at 23 K, then warmed to 150 K. The progressive disappearance of the resonance at 409 eV with the temperature indicates the gradual conversion of HNO3 into NO3-. By fitting the NEXAFS spectra with a linear combination of the HNO3 and NO3- spectra presented figure 1, = the relative abundance of these two species can be estimated as function of the temperature (inset). Although the spectrum at 23 K looks like that of pure HNO3, the fit indicates that actually 25 % of nitric acid is already converted in NO3- (inset, blue circle).
An easy conversion into nitrate
This is a very important result showing that ionic dissociation of HNO3 at the surface of ice may naturally occur with no activation barrier, supposedly when HNO3 sits on a favorable adsorption site, i.e. providing enough water molecules to dissociate HNO3 and solvate the nitrate anion. The rest of HNO3 is molecular, and corresponds to molecules sitting on other surface sites having not such capabilities. These molecules are progressively converted into NO3- when increasing the surface temperature, which provides the necessary energy for the optimization of the solvation shell, leading to dissociation. Around 110 K, the steep increase in the conversion rate is due to the diffusion of nitric acid molecules in the bulk of ice where they find enough water molecules for ionization and solvation.
This study shows that ionization of HNO3 is facile at the surface of ice, and its diffusion into the bulk achieves its complete conversion into NO3-. This indicates that, for atmospheric processes involving the reaction of HNO3 with the surface of atmospheric hydrometeors or wet particulate matter, nitric acid is expected to behave as a strong acid at typical atmospheric temperatures of the Earth’s cryosphere (around 250 K). Yet, it would be interesting to confirm that point in the next future, for instance using the Near-ambient-pressure-XPS (X photoelectron spectroscopy) end-station under development on the TEMPO beamline.