Mpox is an infectious disease transmitted by contact that can be fatal or leave significant sequelae. Scientists at the Institut Pasteur have identified and characterized a new target for developing therapies that neutralize the virus and protect against the disease.
Using structural data obtained at SOLEIL from the POLARIS cryo-electron microscope and the PROXIMA-1 & PROXIMA-2A beamlines, they showed how some neutralizing antibodies block viral entry and used the structural data to generate a new immunogen that elicited protective immune responses in mice.
The monkeypox virus causes mpox, a serious human disease that is closely related to smallpox. Mpox is a zoonosis1 that causes recurrent outbreaks in Central and Western Africa; however, since 2022, two strains adapted to human-to-human transmission have led to unusually large epidemics that have spread across countries and continents. First-generation vaccines used to eradicate smallpox are based on virulent strains and are not considered safe for the current medical standards and the modern attenuated vaccines are expensive and provide only short-term protection. Tecovirimat, the only antiviral available to treat mpox, has been shown to be ineffective in reducing symptoms or the severity of the disease in multiple clinical trials. As a result, we currently lack effective therapeutic tools to contain large-scale outbreaks of mpox, and the WHO declared the disease a public health emergency of international concern.
Scientists at the Institut Pasteur, with the help of several SOLEIL beamlines, are tackling this problem. Early 2025, using data collected at beamlines PROXIMA-1, PROXIMA-2A and SWING, they showed that tecovirimat prevents viral egress by inducing the dimerization of a viral phospholipase termed F13. They also reported the crystal structure of the complex F13/tecovirimat, paving the way to develop novel antivirals.
More recently, they focused on how the virus enters cells. One of the most promising therapeutic targets for developing antiviral treatments are a family of proteins that mediate viral entry called “fusion complexes”, which have been insufficiently studied in poxviruses because of their complexity. In their latest study, the scientists studied A16/G9, a part of the fusion complex of the virus which plays a crucial role in determining which cells to infect and when to infect them. They engineered a stable complex that mimicked the structure of A16/G9 on the viral particle. Then, they used the new cryo-electron microscope POLARIS available at SOLEIL (funded by EQUIPEX and the ANR (ANR-21-ESRE-0046)) to obtain the structure of A16/G9 in complex with another viral complex, which role is to prevent superinfection (i.e., infection of a previously infected cell) (Figure 1). The structure showed how the two viral protein complexes interact, opening up new opportunities for the development of a novel class of antivirals and confirming that the engineered A16/G9 complex acquires a biologically relevant conformation.
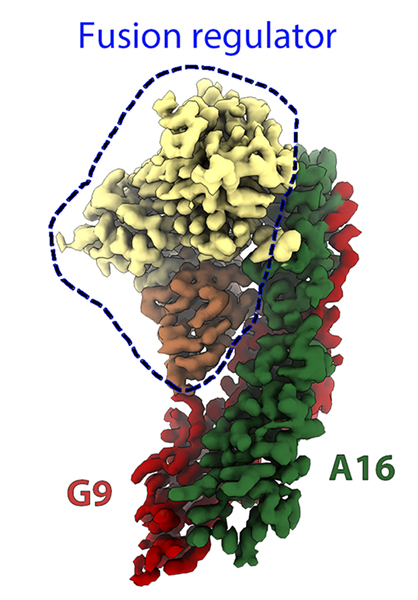
Figure 1: Cryo electron microscopy of A16/G9 (in red and green) in complex with a viral fusion regulator (in yellow and orange).
Next, they injected the A16/G9 complex to an alpaca, whose immune system responded by producing some antibodies that cross-neutralize several poxviruses, including the two mpox strains that can infect humans. To study how these antibodies block viral entry, they crystallized them in complex with A16/G9, collected X-ray diffraction data at the PROXIMA-1 and PROXIMA-2A beamlines, and obtained atomic structures that identified the antibody-binding sites and helped to understand the neutralization mechanisms (Figure 2).
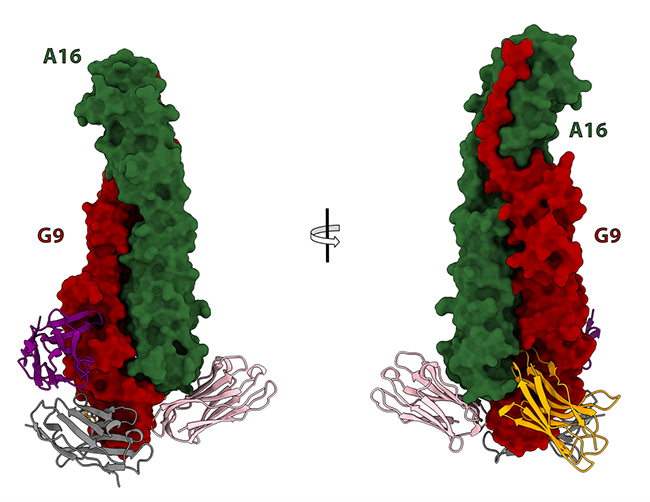
Figure 2: X-ray crystallography structures of A16/G9 in complex with antibodies.
Finally, they immunized mice with A16/G9 and showed that the elicited immune response protected the animals from a challenge with a lethal poxvirus dose, indicating that the engineered complex can be used as a vaccine.
The next step is to evaluate whether vaccines containing A16/G9 elicit better or longer-lasting immune responses than the current vaccine compositions in clinical trials. In the future, they expect that these efforts will serve to contain deadly epidemics caused by mpox or any other poxvirus that may emerge or reemerge.
1 – zoonosis: a disease or infection whose pathogen (bacteria, fungi, virus, parasite or prion) can be transmitted from animals to humans, and vice versa.
