MARS beamline - Relevance of formation conditions to the size, morphology and local structure of intrinsic plutonium colloids brought to light
Anthropogenic activities have released considerable amounts of radioactive “hot” particles into the environment. Predicting their behavior and reactivity therefore requires their thorough characterization in their native environment.
It is within this framework that researchers from the Laboratory for Sonochemistry in Complex Fluids at Marcoule Institute for Separation Chemistry (UMR 5257 – CEA/CNRS/UM/ENSCM), supported by researchers from ICSM, CEA/DMRC and MARS beamline, have combined synchrotron SAXS and XAS techniques to probe on the beamline the multi-scale structural properties of Pu(IV) intrinsic colloidal suspensions prepared by hydrolysis or sonolysis.
Anthropogenic activities including nuclear weapon production and testing, disintegration of satellites equipped with nuclear power sources in the atmosphere, or industrial accidents have released substantial amounts of actinides into the environment. The element plutonium, which was thought to be highly immobile regarding its very low solubility in aquatic systems and high sorption ability onto minerals, has been proved to move on the scale of kilometers. Both pseudo and intrinsic plutonium colloids have been reported to be potentially involved in the environmental migration of actinides, thus strengthening the need for a better description and characterization of their structure. Pseudo-colloids usually refer to natural colloids on which Pu has been incorporated or adsorbed, whereas intrinsic ones relies on the ability of Pu to precipitate and form its own colloid.
Research progress on Pu(IV) colloids is however particularly challenging because of the complex chemistry of the element Pu and the high radioactivity of all its isotopes requiring careful handling.
A rigorous characterization of these species is particularly important since the apparent solubility of the colloids strongly depends on the size of the nanoparticles comprising the suspensions. Furthermore, solubility and interactions with the environment (dissolution, RedOx interactions, adsorption, complexation, …) may be significantly impacted by the nanoparticle speciation and the chemical nature of their surface. Thus, scattering and/or absorption techniques are very useful to probe directly in the synthesis solution the nanoparticles size and morphology (i.e. shape and form), and the nature of the interaction of the particles (local structure, speciation) without any specific sample preparation. This non-intrusive technique providing in situ information averaged over large sample volumes and measurements in the native environment appears therefore ideal for Pu colloidal systems. Two intrisinc Pu(IV) colloidal forms prepared by hydrolysis of Pu(IV) in pure water or sonolysis of PuO2 nanopowder in water under Ar/(10 %)CO atmosphere were investigated.
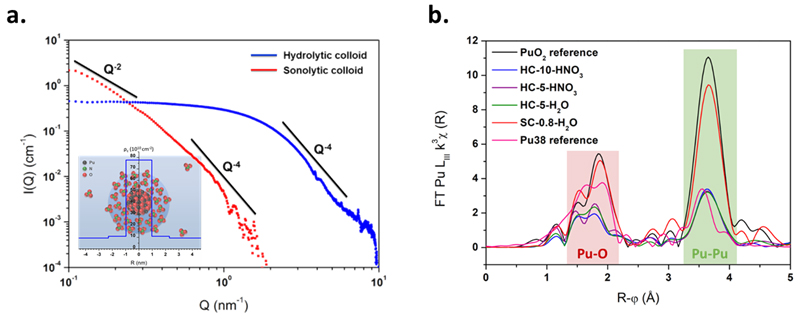
The normalized SAXS diagrams measured for both hydrolytic and sonolytic colloids are given in absolute unit in Figure 1. Best fit for hydrolytic colloid diagrams were obtained using a spherical core-shell model where the colloid core was assumed to have an ideal PuO2 fluorite-type Fm-3m oxide structure surrounded by a disordered Pu-O shell solvated with nitrate counter-ions. Sonolytic colloids are characterized with elongated bigger particles of ca. 5.7 nm thickness and more than 30 nm size (lamella/disk). Whereas hydrolytic colloids were found to agree with previous HR-TEM experiments, the difference observed for sonolytic colloid (10 nm by HR-TEM) may results from the synthesis mechanism and be attributed to nanoparticle aggregation in the absence of capping-ions. The Pu LIII edge XANES spectra acquired for a selection of hydrolytic and sonolytic colloid samples support the (+IV) oxidation states for the samples investigated in this study. In comparison to reference spectra of “bulk” PuO2 and a cluster of Pu, EXAFS analysis points out two distinctive features for both hydrolytic and sonolytic colloid samples: i) a reduced oscillation amplitude for both Pu-O and Pu-Pu coordination shells, and ii) a strong asymmetry and distortion of the Pu-O coordination sphere (figure 2). Both effects are found significantly correlated to the nanoparticle size probed by SAXS and strengthened for the sonolytic colloid whose spectrum appears intermediate between the hydrolytic ones and the PuO2 reference.
These combined synchrotron-based SAXS and XAS measurements provides new insights about the local structure, size, morphology (i.e. shape) and interfacial properties of intrinsic Pu(IV) colloids, that could help in a better understanding of Pu migration mechanism in the environment. Future investigations with different salt systems and more concentrated solutions should strengthen this approach and clarify the plutonium colloid shell nature.
(a.) SAXS diagrams of 10 mM hydrolytic (blue) and 0.8 mM sonolytic (red) intrinsic Pu(IV) colloids. Black lines indicate the slope power laws. Insert: Schematic representation of spherical colloid composed of a PuO2 core surrounded by shell containing nitrate ion in bulk solution.
(b.) Fourier transforms (FT) of the k3-weighted 2.5-15 Å-1 interval for a selection of hydrolytic and sonolytic Pu(IV) intrinsic colloids framed with additional FT from bulk PuO2 (black) and simulated Pu38 cluster (pink).