Long-range attraction between hydrophobic surfaces - Direct measurement of lateral correlations under controlled nano- confinement
While the hydrophobic effect forms the basis for many chemical and biological phenomena, the interaction between two hydrophobic substrates is still not understood. Thanks to the development of an ultra-sensitive experimental instrument that combines the techniques of the surface force apparatus (SFA) and small angle scattering, researchers of the Charles Sadron Institute (ICS) of the University of Strasbourg and the SIRIUS beamline have been able to decipher the mechanism of attraction between hydrophobic macroscopic surfaces.
One of the questions that remains concerning the interaction existing between water and surfaces is the nature of the area of contact between the water and a hydrophobic surface, this being key to understanding the hydrophobic effect. Hydrophobic interactions play an important role in numerous biological phenomena such as protein folding and self-association of surface-active agents (surfactants) and lipids in solution. Moreover, they are used in numerous applications, extending from detergency and dispersant properties, formulations (emulsions, creams, shampoos, colorants, etc.), nanoparticle synthesis, through to lubrication and the assisted recovery of ores (flotation). A large number of works have suggested that the density of water is reduced at the hydrophobic substrate/water interface and that the water film undergoes significant dewetting in the contact region (cavitation) when two hydrophobic substrates are brought close to each other. However, the microscopic details of how the water comes into contact with a hydrophobic substrate were still not firmly established. So much so that the mechanism which would explain the observation of the attraction between hydrophobic macroscopic surfaces, both long-range (of the order of a hundred nanometres) and of amplitude two to three orders of magnitude greater than the van der Waals interaction, had remained without a satisfactory explanation for more than 40 years.
The ability to resolve this fascinating question requires the ability to investigate the interfacial region not only in the vicinity of the hydrophobic substrate in contact with an aqueous solution, but in particular to be able to track its structural development when the aqueous film confined between two such hydrophobic substrates is progressively reduced in a controlled manner down to the nanometric or sub-nanometric scale. This is an experimental challenge.
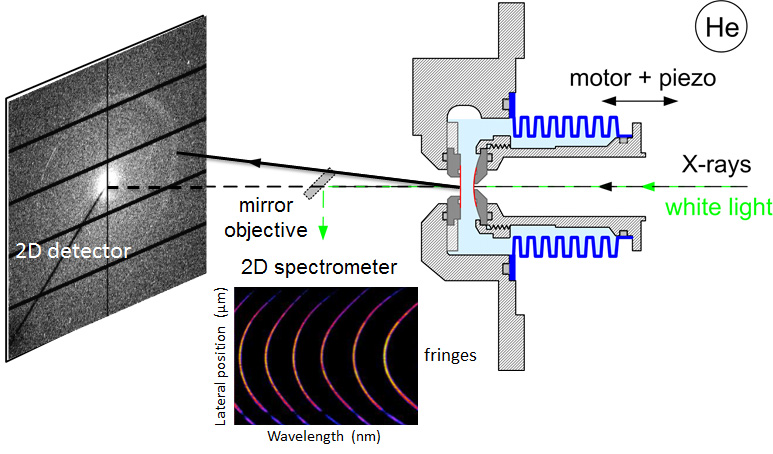
Thanks to an advanced and ultra-sensitive instrumental development, which has occurred over the course of more than ten years of continuous effort, scientists from ICS and the SIRIUS beamline have managed to measure the structure in the confined space (water film confined between two hydrophobic substrates) using the beamline with diffuse scattering-diffraction of X-rays, while varying the spacing between the two hydrophobic substrates in a continuous and controlled manner at a sub-nanometric scale. To put it another way, this experiment couples the technology of the surface force apparatus (SFA) with that of small angle X-ray scattering, named SFAX, which can be seen as a microfluidics experiment only with the advantage of being continuously adjustable and finely controllable from which the structure of the confined film can be determined.
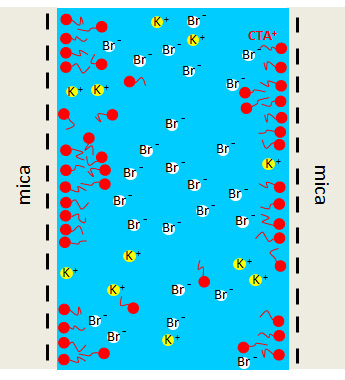
It appears that the long-range attraction between two hydrophobic charged neutral macroscopic surfaces originates in the lateral correlations of the positions of the ions in solution along the surfaces. These correlations are formed and strengthened when the two substrates are brought closer together. It is this redistribution of ions in the planes of the surfaces, which creates an attractive force in the perpendicular direction. Everything comes down to a balance between the two characteristic lengths of the system effective in the two directions (lateral correlation length versus the separation along the normal), which synergise to create an electrostatic-type attraction in an aqueous medium.
The reliability of the SFAX apparatus demonstrates the advantage of this type of coupled measurement. It opens up a huge unexplored field applicable to condensed matter physics, physical chemistry and also biology with multiple applications in these materials. It will also be possible to study specific directional interactions of very short range (below 20 nanometres) which is the foundation of biological recognition, nanoparticle conformations, aggregates of surfactants, polymers and copolymers subject to confinement (transition from 3D to 2D), phase transitions induced by confinement, and also the nano-structuring of ionic liquids close to metallic electrified interfaces, the understanding of which is important for energy storage for example.