Ionization potential of the cyano radical and implications for CN+ cation chemistry in space
The cyano radical (CN) is omnipresent in many reactions and environments (plasma, combustion, …). It is for instance one of the first species detected in astrophysical media such as comets (in 1881) or even diffuse clouds. However, despite its relevance, its ionization potential was not precisely known until recently.
Researchers from the Institute of Molecular Sciences in Orsay, the SOLEIL facility, the Institute of Physical and Theoretical Chemistry of Würzburg and the Institute of Molecular Sciences in Bordeaux have for the first time been able to measure directly the ionization potential of the cyano radical on the SOLEIL DESIRS beamline, thanks notably to the DELICIOUS III double imaging (photoelectron-photoion coincidence) spectrometer.
In the regions of space dominated by photodissociation, the CN+ + CO -> CN + CO+ charge transfer reaction plays an important role in nitrogen chemistry. The enthalpy of this reaction can be deduced from the ionization potential (IP) of CN and CO species. Even if the IP of CO has been measured very precisely, the IP of CN was poorly known and suggested that this reaction was very favourable even at very low temperatures.
Thanks to an experiment combining an original gas-phase radical source, synchrotron radiation and a double imaging (ion/electron) detection technique, the CN IP has for the first time been accurately measured. The results show that the CN+ + CO -> CN + CO+ charge transfer reaction is actually very slow at low temperatures.
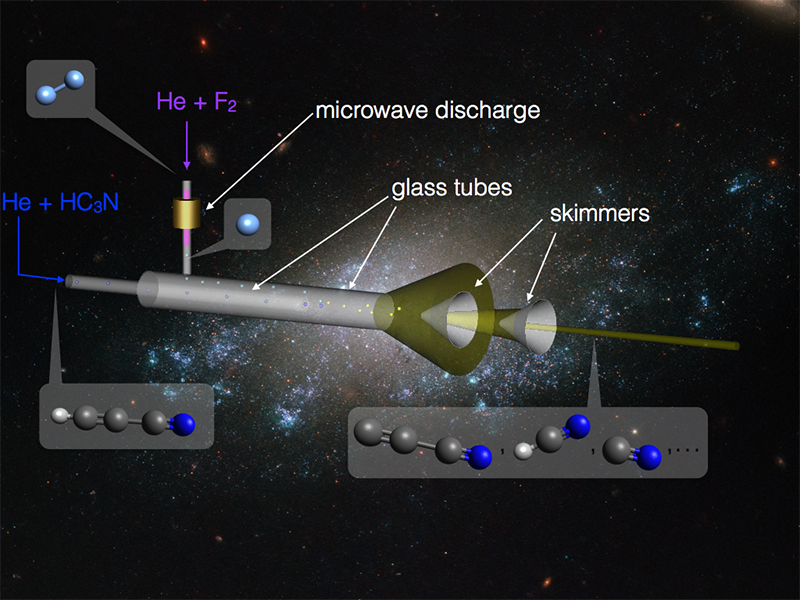
The cyano radical was produced in the gas phase in a flow tube incorporating an atomic fluorine source. The principle of this source is based on the high atomic fluorine reactivity with hydrogenated species. It forms HF from a precursor by capturing its hydrogen atoms to efficiently produce radical species. In this study, we used cyanoacetylene (HC3N) to produce the C3N radical, which, in the flow tube, reacts with the remaining precursor to form hydrogen cyanide (HCN), which in turn reacts with the atomic fluorine to produce the CN radical. A diagram of the radical source is presented in Figure 1.
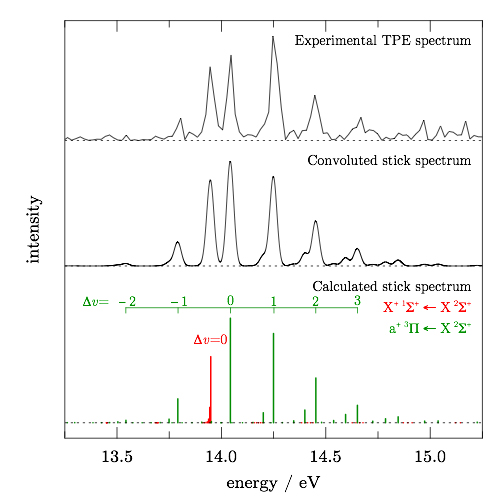
The produced species are subsequently ionized by monochromatic VUV radiation from the DESIRS beamline. The ions and electrons that are formed are then analysed with the DELICIOUS III double imaging (photoelectron-photoion coincidence) spectrometer, which is capable of providing simultaneously the photoelectron spectroscopy of any and all the masses present in the flow-tube reactor, with a high signal-to-noise ratio. The threshold photoelectron spectrum of the cyano radical is presented in Figure 2.
In order to assign the vibronic transitions observed in the threshold photoelectron spectrum, the researchers performed ab initio calculations on the neutral CN radical and its cation. The very good agreement between the simulated and the experimental spectra enabled them to unambiguously determine the adiabatic ionization energy of CN. The comparison of the calculations with the experimental spectrum as well as the assignment of the observed bands are presented in Figure 2.
This experiment represents the first direct observation of the photoionization transitions of the CN radical and, combined with theoretical calculations, provides important information on this radical and its cation. Notably, it has been possible to demonstrate that the ionization potential of CN is slightly lower than that of the CO molecule, which implies that the CN+ + CO -> CN + CO+ reaction is endothermic at low temperature.
This result calls into question previous assumptions about this reaction in photochemical models of astrophysical environments at low temperatures.