Induced chirality onto an achiral molecule also exists in Photoelectron Circular Dichroism (PECD)
Circular Dichroism measurements have already demonstrated that an achiral(1) molecule can acquire a chiral(1) spectroscopic signature when interacting with a chiral environment. A new study, conducted at the beamline DESIRS, shows that when an achiral molecule is in interaction with a chiral one, its exposure to polarized VUV(2) radiation leads to an asymmetric scattering of the ejected photoelectrons(3).
This demonstrates that induced chirality can also be observed in photoelectron circular dichroism (PECD(4)). Such an induced chirality has important structural and analytical implications, in the context of growing interest in laser-based PECD, for in situ, real time enantiomer(1) determination.
Induced chirality refers to the appearance of a dichroic signal onto an achiral molecule embedded in a chiral environment. Such a long-range effect has been observed in the past via various chiroptical spectroscopies, such as electronic or vibrational circular dichroisms, both based upon an electric and magnetic dipole interplay, leading intrinsically to weak dichroic asymmetries.
In contrast, PhotoElectron Circular Dichroism (PECD(4)) is a chiroptical spectroscopic effect allowed in the electric dipole approximation, leading to orders of magnitude larger asymmetries. Observed as a forward-backward asymmetry in the photoelectron angular distribution after ionisation of a chiral system by Circularly Polarised Light (CPL(5)), it depends on both the initial state, i.e. the orbital from which the electron is ejected, and the final state, i.e. the electron continuum and therefore on the kinetic energy of the outgoing photoelectron scattered by the chiral potential of the cation. As such, it is very sensitive to molecular conformation and to aggregation.
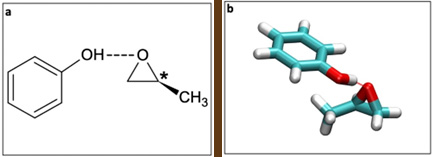
The range of PECD is still an open question, with strong effects measured from achiral orbitals not located at the chiral center. These studies, however, have been limited to intramolecular distances (1 or 2 Å), for both valence- and core-shell PECD. Here, a collaborative consortium based upon a team from ISMO (CNRS / Univ. Paris-Saclay) and the DESIRS beamline team focused on the weakly-bound molecular complexes formed between phenol (Phe), a non-chiral chromophore, and methyloxirane (MOx) (cf Fig.1). They aimed to assess the range of the influence of the chiral host on PECD across intermolecular distances, potentially leading to an induced PECD onto the achiral moiety.
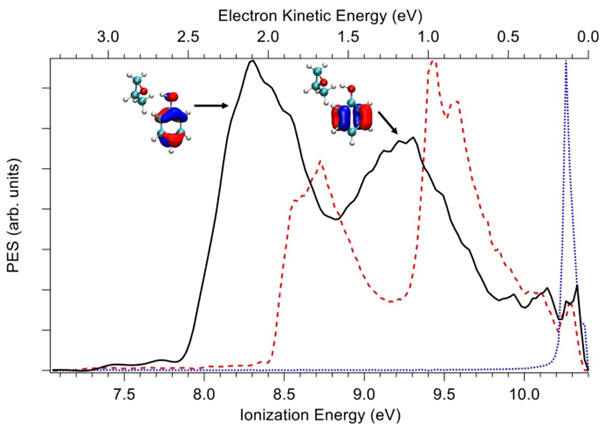
By using the electron/ion double imaging coincidence spectrometer available on DESIRS, the scientists recorded the Photoelectron Spectrum (PES(3)) of the Phe-Mox complex showing two clearly-resolved bands, corresponding to the two outermost orbitals (HOMO and HOMO-1) both purely localized on the aromatic achiral p-orbitals of the phenol moiety (see Fig.2).
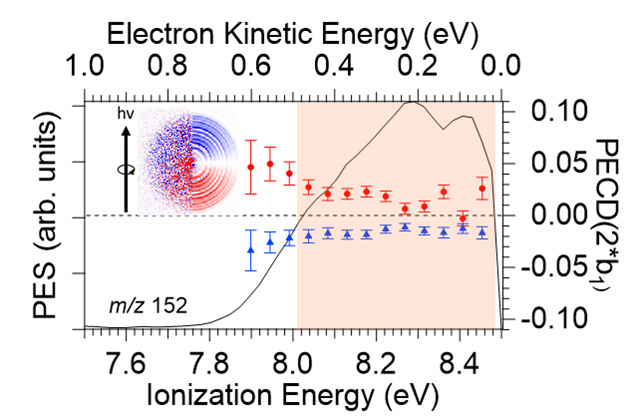
At all examined photon energies, and in particular at 8.5 eV, where the chiral MOx cannot be ionised, a clear PECD-asymmetry is evidenced as visible in Fig. 3, with the expected mirroring between the complexes of phenol with the (R) and (S) enantiomers of MOx. The origin of the induced PECD observed on the achiral HOMO orbital of the complex should stem from the chiral potential that is experienced by the scattered photoelectron. This first example of the manifestation of induced chirality in PECD on an achiral chromophore by complexation with a chiral tag underlines the role of the long-range chiral scattering potential extending to intermolecular distances, here up to ~5 Å. In other words, from the point of view of the achiral moiety, the presence of the chiral MOx host offers to the phenol departing electron a chiral scattering potential, leading to induced PECD.
Conversely, from the point of view of the chiral moiety, induced PECD on the achiral chromophore offers innovative analytical opportunities. Indeed, the first demonstrations of laser-based multi-photon (MP) PECD in 2012 (a decade after the first experimental evidence with Synchrotron Radiation), triggered a blooming of the field fuelled by the high potential of MP-PECD as a table-top analytical tool to retrieve in situ enantiomeric excesses in the gas phase. However, because of the ionization scheme used involving near/mid-UV range photons, these studies have been so far limited to terpenes or to chromophore-bearing species. Complexation with an achiral chromophore could be a way to extend such laser-based chiroptical measurements to a large array of chiral species which cannot be excited in the near/mid UV, including for instance non-aromatic amino-acids, whose PECD has been so far studied only by one-photon synchrotron ionization, a technique which unlike laser MP-PECD, does not allow a direct selection of conformers(6).
-------------------
GLOSSARY
1 - Chirality / enantiomer: An object is “chiral” if it exists as two non-superimposable forms which are mirror image of each other. This is the case of hands (or feet!), propellers, but also of many molecules (for example, the ones with an asymmetric carbon linked to 4 different chemical groups) – the two forms are called left or right enantiomer.
2 – VUV: vacuum ultraviolet, ultraviolet radiation absorbed by air. Corresponds to the 30-200 nm wavelength range (6-40 eV), ie the excitation of valence shell electrons of atoms and molecules.
3 – Photoelectron & photoelectron spectroscopy (PES): light can tear off an electron from matter, it corresponds to the photoelectric effect: the energy of the incident photon (hn) is shared between extraction work of the electron (binding energy) and the kinetic energy of the emitted electron (Ek), so-called “photoelectron”. Photoelectron spectroscopy measures the distribution of Ek energies for a given incident photon energy, allowing the determination of the (binding) energy levels of the electrons in the molecule (which corresponds to the molecular orbitals energies). One can also measure in which directions the photoelectrons are ejected with respect to a given axis: i.e angular distributions. This distribution (characterized by the b parameter) does not have the same properties in all directions, in other words: is not isotropic (b ≠ 0) because the molecule "remembers" the polarization of the incident light.
4 - PhotoElectron Circular Dichroism (PECD): As Circularly Polarized Light (see below) is a chiral object, it will induce enantio-specific processes upon interaction with a given enantiomer of a chiral species: these are circular dichroisms, a chiral recognition between chiral objects, such as a left glove "recognizes" a left hand. In the case of photoemission of a given enantiomer of a chiral molecule by an CPL of a given helicity (left or right), the PECD manifests itself by a forward / backward asymmetry with respect to the light propagation axis, which is not found for non-chiral species. This asymmetry is reversed when the enantiomer or light helicity is swapped. The specificity of PECD, as compared to other types of circular dichroisms, lies in its intensity: from a few % to a few tens of %. It is also very sensitive to molecular structures such as isomers (molecules with the same atomic composition, but a different arrangement of these atoms), or conformers.
5 - Circularly Polarized Light (CPL): Polarization is a geometric property of light, which describes the motion of the electric field associated with the light wave as it propagates. In the case of circularly polarized light, the electric field describes a helix (like a corkscrew) which can be clockwise or counterclockwise, defining then CPL with left or right helicity. Note that CPL is a chiral object.
6 - Conformers: many molecules and in particular biomolecules, are floppy, existing as several conformers, obtained by rotations of chemical groups around a chemical bond and by deformation (puckering) of a ring.