Immunity - X-ray diffraction reveals protein interactions at the heart of the defense against pathogens
The immune system is constantly identifying and destroying pathogenic bacteria, viruses and other factors of infection. The complement system is one of the immune system’s components, comprising numerous proteins. Two laboratories of the Center for Biological Research Margarita Salas (CSIC, Madrid) and the team of PROXIMA-2A beamline have succeeded for the first time in unraveling the 3D structure of iC3b, one of the complement proteins, as well as that of iC3b linked to a complement receptor protein, CR3. The structures obtained contribute to a better global understanding of the pathogen labelling processes for their elimination, in which iC3b and CR3 are key players.
To protect us against the various pathogens to which we are constantly exposed, our immune system uses various players and mechanisms, which either require time to adapt to be active (adaptive immunity - including the production of antibodies) or allow for immediate defense (innate immunity). The complement system is one of the innate immunity players. It is composed of more than 30 proteins that act sequentially: one protein activates another, which activates a third, and so on - hence the name "complement cascade". One of the functions of the complement system is to help eliminate cells that are not recognized as part of our body by labelling them with specific signaling molecules (opsonins), which then alert other actors in the immune system to the foreign nature of these pathogens. Sometimes the opsonins themselves destroy the pathogen by creating pores in the surface of its cell. More often, however, they rely on other immune cells to phagocytose the pathogen.
The central component of the complement system is a large multidomain protein called C3, one of the most abundant proteins in the blood, whose structure and function have been studied for years. Its main function is to bind and permanently mark 'intruder' surfaces and pathogens. When C3 deposits on these targets, it undergoes a conversion that transforms it into a closely related protein with a radically different structural organization, called iC3b. C3 deposition occurs so fast and so completely that within minutes the pathogen is surrounded by a maze of iC3b molecules. From this point forward, the immune system can then detect all sorts of pathogens by recognizing the presence of just one molecule, iC3b. Although the importance of iC3b for the immune system has been recognized for many years, its structure has remained elusive, surrendering only low-resolution images from negative-stain electron microscopy or small-angle X-ray scattering.
Two laboratories of the Center for Biological Research Margarita Salas (CSIC) in Madrid, Spain with the help of the PROXIMA-2A beamline team at SOLEIL have collaborated to unravel the complete structure of iC3b by X-ray crystallography. Their work reveals the modular organization of iC3b, characterized by a globular domain that mediates cell-surface attachment flexibly connected to a rigid body by a ~65-Å tether.
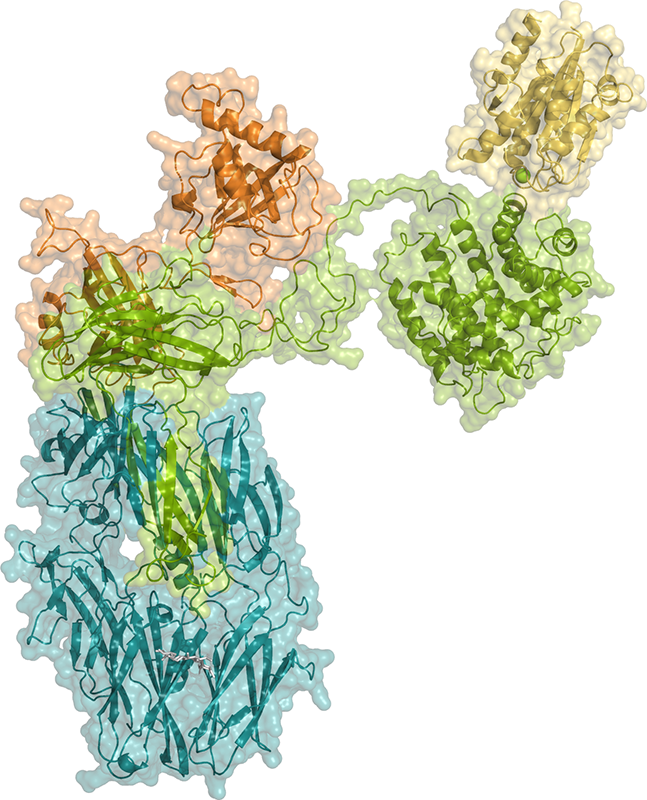
Furthermore, the structure of a complex between iC3b and the CR3 protein was also determined (Figure 1). CR3 is a cell-surface receptor present in most immune cells, including the macrophages and neutrophils responsible for eliminating pathogens by phagocytosis (CR3 = complement receptor 3), to which iC3b binds. The structure of the complex between iC3b and a domain of CR3 - called αI - shows how the modular structure of iC3b plays a crucial role in creating the surfaces required for CR3 binding. Since iC3b is attached to the pathogen surface and CR3 to the immune-cell surface, the complex models the dense cell-cell interactions required for efficient recognition and destruction of pathogens by the immune system.
The crystals obtained from the iC3b-CR3 αI complex were long and fragile, and it was difficult to collect usable X-ray diffraction data. However, the PROXIMA-2A beamline scientists were able to obtain high-quality X-ray diffraction data that reached a resolution of 3.4 Å by exploiting the properties of the PROXIMA-2A X-ray microbeam with a careful data collection strategy.
This work highlights the role of the complement system in labelling the surface of pathogens so that they are recognized by specific receptors present on the surface of macrophages and other immune cells (and then destroyed). The structure of the iC3b-CR3 αI complex reveals the molecular mechanisms underlying these processes, which are essential for the correct function of the immune system.