Hydrogen atoms kick molecules off the surface of water ice
One of the possible outcomes of the excitation of a surface by energetic particles is the desorption of molecules adsorbed on that surface. This phenomenon has been an object of fundamental study in surface science for decades. Yet, for only few systems it has been possible to elucidate the exact molecular-scale mechanism through which the incoming energy of the particle is converted in the motion of molecules and them eventually leaving the surface.
A team from the Laboratoire d’Etudes du Rayonnement et de la Matière en Astrophysique et Atmosphères (Sorbonne Université), on collaboration with researchers from the Institut de Chimie Physique (Université Paris-Saclay), CERN, and Laboratori Nazionali di Frascati, conducted experiments that led them to prove which mechanism leads to photon-induced desorption of light molecules at the surface of water ice, a particularly important and interesting surface. Photons break up water molecules, expelling energetic hydrogen atoms that collide with the adsorbed molecules, expelling them through a momentum transfer that can be accounted for by an unexpectedly simple model.
Photon-induced (but also electron- and ion-induced) desorption from water ice has been the object of a considerable amount of works, owing to the relevance of water ice in many contexts. One example of these contexts is astrochemistry. In the cold star- and planet-forming regions of the universe, molecules condense at the surface of tiny dust grains and form so-called ice mantles mainly composed of water. As long as these grains remain away from heat sources, molecules should stay trapped at their surface. However, non-thermal processes intervene to release some of these molecules to the gas phase, as evidenced by gas phase observations that cannot be explained otherwise. Hence the importance of studying processes such as photodesorption in this context.
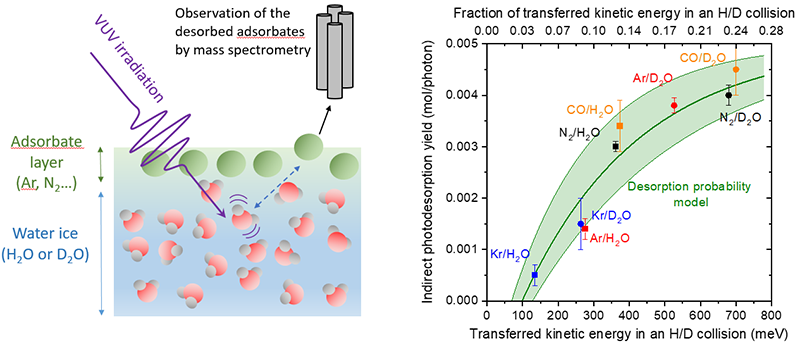
Multiple mechanisms have been proposed by both theoretical and experimental studies to explain desorption from the pure water ice. Despite these efforts, no clear experimental evidence has been reported that can single out one particular mechanism. To test some of the hypothesis that have been discussed, the authors of the study instead looked at a system slightly different from the pure ice: small atoms and molecules adsorbed at the surface of water ice. Fig. 1 shows on the left a simple schematics of the principle of the experiment, where one atomic/molecular layer of a species (Ar, Kr, CO or N2) is deposited on top of regular water ice (H2O) or deuterated water ice (D2O). This system is irradiated with the synchrotron beam and the efficiency of photon-induced desorption of the adsorbed species is monitored. In this context, the use of synchrotron radiation is crucial: these experiments require a monochromatic photon beam tunable over a wide range in the VUV (7-14 eV), in order to make sure that only water ice is indeed excited. In addition, these difficult experiments require a high enough photon flux, obtained from the undulator of the DESIRS beamline at SOLEIL.
A systematic study of the efficiency of desorption of the different adsorbed species by water excitation reveals a trend that allows to conclude on the mechanism. The way this mechanism operates is quite simple: first, water excitation leads to dissociation of the molecule. The excess energy is for a large part taken away by the hydrogen atom. This hydrogen atom then collides with species adsorbed at the surface and transfers them some of its momentum, which if sufficient can lead to desorption. Hence the name: kick-out. This simple mechanism can be modelled in first approximation by a hard sphere collision, a model which only depends on the mass of the two colliding objects. It is thus possible to calculate, for each adsorbate (N2, Ar, etc) and each ice (H2O or D2O, D atoms being twice heavier than H atoms) what fraction of the momentum would be transferred from H/D to adsorbate. A more sophisticated, semi-empirical model along with some energetics considerations translates this momentum transfer into a desorption probability. The agreement between this rather simple model and the experimental data is presented on the right panel of fig. 1. It is very good considering the uncertainty inherent to such low-yield measurements, the simplicity of the model, and the fact that it is applied at the molecular scale, to objects quantum in nature and susceptible to many other phenomena such as reactive collisions.
The elucidation of this mechanism is a step forward in the fundamental understanding of surface desorption phenomena, but also helps in modelling these processes in the various domains where they are relevant.
This work benefited from the SOLEIL synchrotron facility at the DESIRS beamline (project 20180060). It was supported financially by the Programme National “Physique et Chimie du Milieu Interstellaire” (PCMI) of CNRS/INSU with INC/INP co-funded by CEA and CNES, by the LabEx MiChem and the DIM ACAV Région Ile-de-France program, and the European Organization for Nuclear Research (CERN, KE3324/TE).