The forth way to dissociate
Dissociation is one of the simplest chemical reactions, where a molecule breaks apart into two or more fragments, i.e., other molecules, atoms, ions, or radicals. Understanding the mechanisms of dissociation of reactions is essential as this knowledge can be the guide to the complex world of biomolecular processes.
Yet dissociation pathways may remain elusive in larger systems, constituted of more than three atoms. Until recently only two dissociation mechanisms were known: (1) stretching a bond until it breaks; (2) dissociation over a potential energy barrier through a so-called transition state, where electrons are rearranged so that the old bonds are broken and new ones are formed. Recently a third dissociation modality was discovered – (3) ‘roaming’ reactions, which don’t follow the conventional mechanics of transition state theory. In this last type of chemical reactions, one atom wanders, or “roams”, around the molecular fragment until it finds another atomic partner to make a bond with, and leaves the molecule.
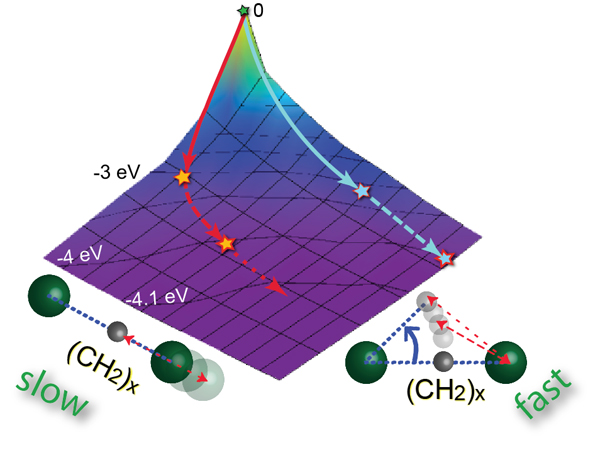
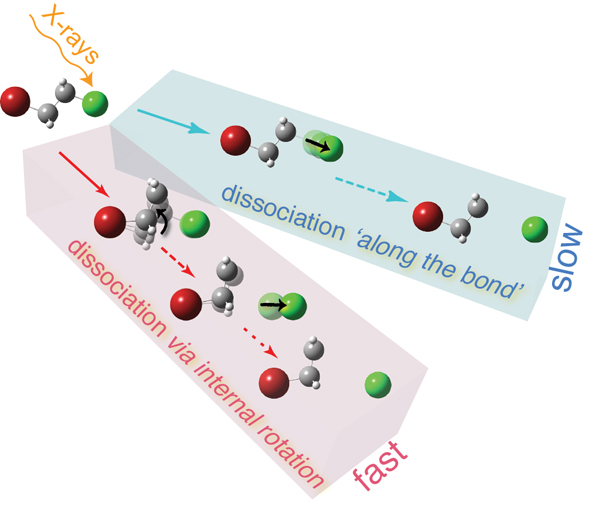
Now the international research group of PLEAIDES beamline has discovered a new dissociation mechanism, which doesn’t fall into any of the three categories of dissociation reactions described above. They infer that in some cases stretching of the bond can be not that simple, as anticipated. The breakage of the bond can be mediated by the internal motions of other lighter atom groups of the molecule, the bond thus being broken not strictly along the bond axis. This allows yielding heavy fragments on very short timescales, by far faster than the classical two-body dissociation would predict. They demonstrate that in their prototypical molecular system – 1-bromo-2-chloroethane (Br-CH2-CH2-Cl, BCE) – just the rotation of the light-weight group of carbon and hydrogen atoms in the middle of the molecule (-CH2-CH2-) around a halogen atom (Br/Cl) may result in the breakage of the carbon-halogen (C-Cl/C-Br) bond. In reality, both the -CH2-CH2-rotation and the C-Cl (or C-Br) bond stretching occur simultaneously. However, due to the considerably lower masses of the carbon atoms relative to that of the halogen (Cl/Br) atom, the rotation is very fast and, therefore, extremely important in the total dissociation process. As shown in the figure below, which represents the electronic surface of the neutral hot BCE molecule, produced by absorption of X-rays, the blue path would be followed if the molecule were to dissociate in a ‘classical way’ – the halogen atom were to separate from the residual molecular fragment by moving apart along the C-Br/C-Cl bond. However, there exists a faster (red) slope from the top of the mountain on this electronic surface, if a third dimension, i.e. -CH2-CH2-rotation, is taken into account. The researchers expect this new dissociation mechanism to be rather general and applicable to other complex molecular systems, especially to the ones with light (-CH2-)x-linkages. Other dimensions could be important to consider while modeling dissociation reactions to predict proper reaction kinetics and product outcomes.
.