Formation and decay of solvated dielectrons observed for the first time
In liquids such as water or liquid ammonia, solvated electrons exist in isolated form, strongly confined within cavities formed in the solvent and, according to various scientific hypotheses, they can also associate in pairs (dielectrons) under certain conditions. So far, however, such solvated dielectrons are the subject of much speculation and have never been observed directly.
Recent experiments carried out on the DESIRS beamline provided direct evidence, supported by quantum chemical calculations, for the formation of these electron pairs by excitation with ultraviolet light in tiny ammonia droplets containing a single sodium atom. The results are published in Science.
Solvated electrons are best known in solutions of alkali metals in liquid ammonia, but have also been observed in other systems. Alkali ammonia solutions are used for many chemical reactions on large industrial scales; mostly for the high chemical reactivity of the solvated electrons, acting as a strong reducing agent. In other solvents like water, solvated electrons occur after exposure to ionizing radiation, and can be harmful (genotoxic) for biomolecules, contributing strongly to radiation damage processes in biological materials.
In alkali ammonia solutions, the existence of solvated dielectrons could previously be inferred from the concentration-dependent magnetic properties of the solutions. Due to the similarities between most other properties of solvated electrons and dielectrons, no other experiments could distinguish the two species. Therefore, most knowledge about solvated dielectrons so far comes from theoretical calculations that could not be verified experimentally.
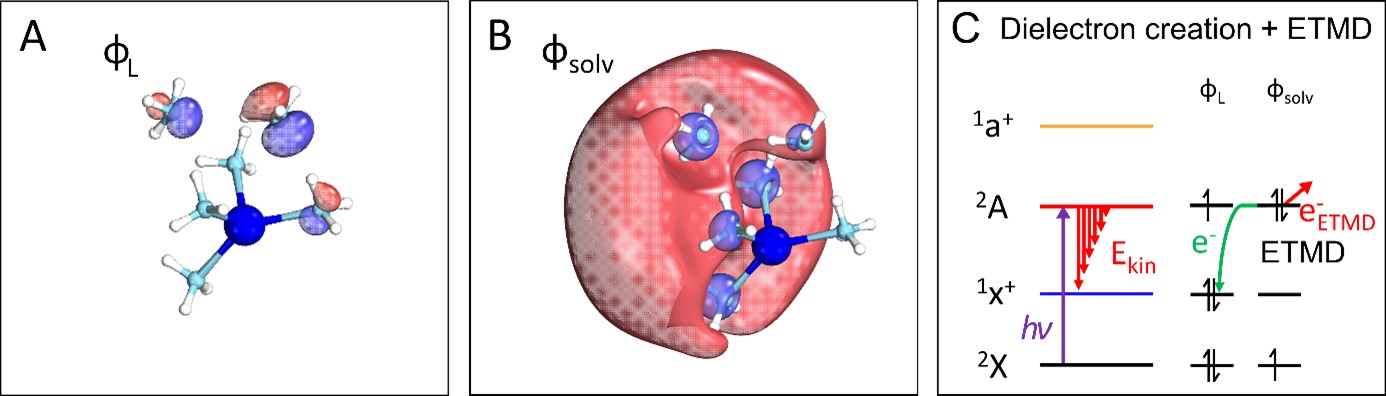
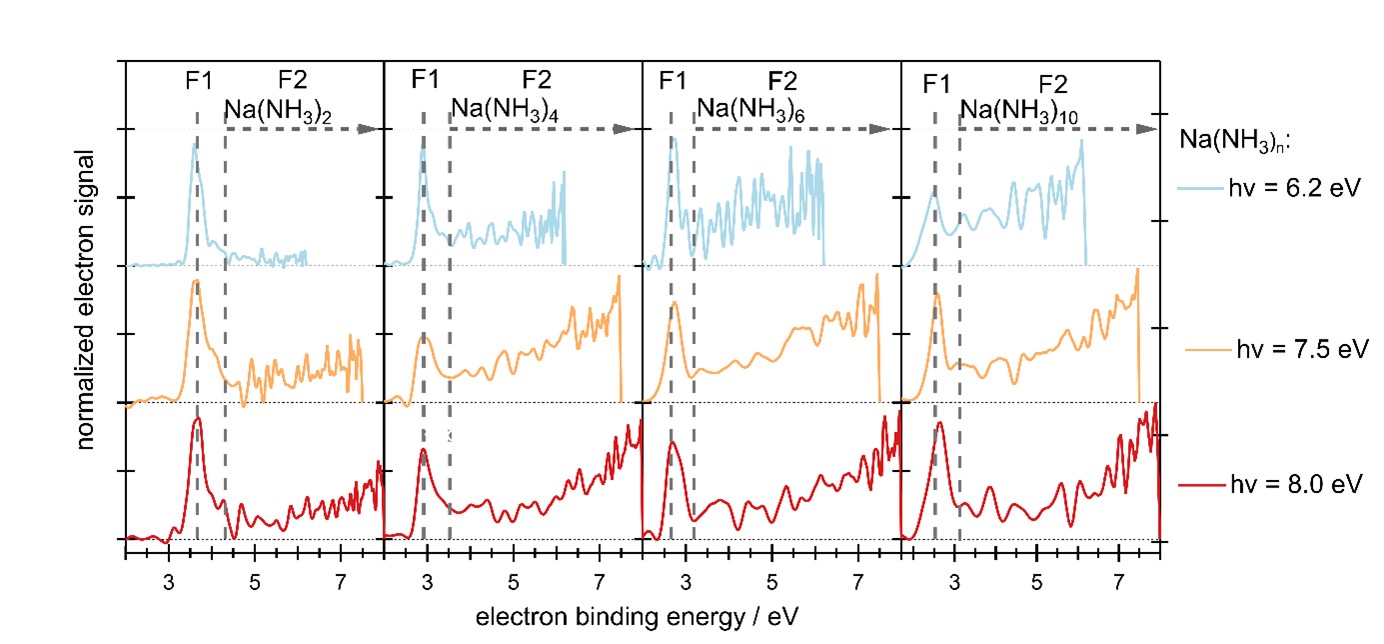
In a recent experiment on sodium doped ammonia clusters Na(NH3)n, performed with the DELICIOUS III electron/ion coincidence spectrometer on the DESIRS beamline, scientists from SOLEIL, the university of Freiburg (Germany), ETH Zurich (Switzerland) and Auburn University (USA), were able to induce the formation of dielectrons by using UV/VUV light to transfer one electron from an ammonia molecule (see figure 1A) to a previously existing single solvated electron (see figure 1B). In this excited state, the orbital of the solvated electron is occupied by two paired electrons, the so-called dielectrons. The formed electron pairs relaxation leaves a unique signature in the photoelectron spectra, when one of the two electrons transfers back to the ammonia molecule (the solvent), while the other is ejected from the cluster with low kinetic energy.
Due to this process, called an electron transfer mediated decay (ETMD, depicted in figure 1C schematically), the signature of the dielectrons in the photoelectron spectrum differs strongly from the signature of single solvated electrons (see figure 2). Similar ETMD processes have so far mainly been observed at significantly higher photon energies, in the XUV to soft X-ray range. In agreement with quantum-chemical calculations, solvated dielectron signatures have been observed over a wide range of UV/VUV photon energies and for all different examined cluster sizes (1-20 ammonia molecules), suggesting that identical processes may occur in bulk solutions.
These results constitute the first direct signatures of solvated dielectrons and a special and thus interesting example of an electron transfer mediated decay process. Furthermore, the observed process could be used to create slow electrons in a controlled way, by using UV light producible by commercially available UV lamps. Electrons with controlled energy may be used for more detailed studies of the reactivity of solvated electrons in alkali ammonia solutions and other solvents.
Such fundamental studies may finally help to improve our understanding of basic reaction mechanisms and help develop alternative chemical reaction pathways working with less dangerous and environmentally harmful reactants.