Electron scattering in liquid water - a molecular picture provided by gas phase water clusters
Electron scattering processes upon photoionization of liquid water and the subsequent transport of the quasi-free electrons through the liquid are of outmost importance for radiation damage in living organisms. However, experimental investigations have so far not been able to provide any molecular-level picture of these processes. Scientists at the ETH Zurich and SOLEIL could recently shed more light onto this topic using an experimental method that allows the measurement of cluster-size resolved scattering features.
Slow electrons with kinetic energies of less than 50 electron-volts (eV) interact strongly with the electronic and vibrational modes of liquid water. Particularly complicated is the scattering behavior of electrons with less than 10 eV kinetic energy. In contrast to high energy scattering, this scattering regime cannot be described by universal models and accurate data can nowadays only be obtained from experiments. Such slow electrons are important for a better description of radiation damage processes in biological media, where secondary electrons with kinetic energies below 10 eV are released abundantly after interaction with ionizing radiation.
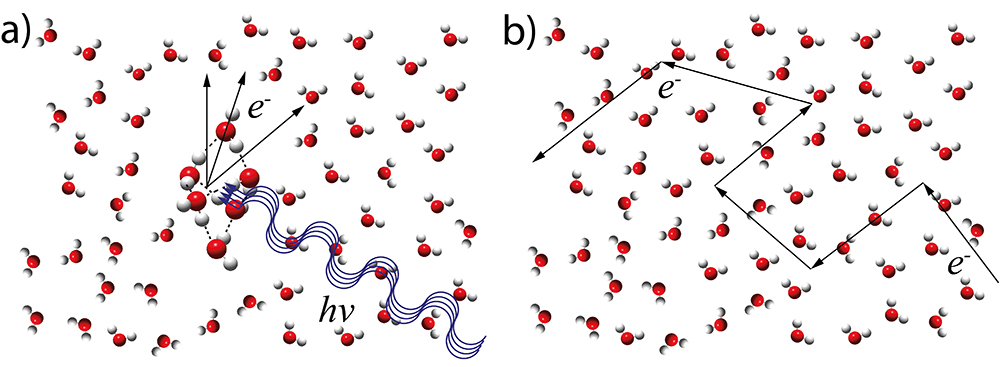
In the focus of the investigation were electron scattering processes upon photoionization with photon energies of up to 35 eV. It was suggested that at these photon energies essentially two different types of electron scattering processes take place in liquid water (Fig. 1):
1/ the photoionization itself, meaning the formation of unbound electrons in the conduction band by photoexcitation of valence electrons (Fig. 1a).
2/ the scattering of the unbound electrons during their transport through the liquid (Fig. 1b).
The description of the second process has recently been solved by a detailed electron transport model by the ETH team. The description of the first process from liquid water data, however, is a difficult one since these data do not provide information on the number of molecules involved.
Size-specific information
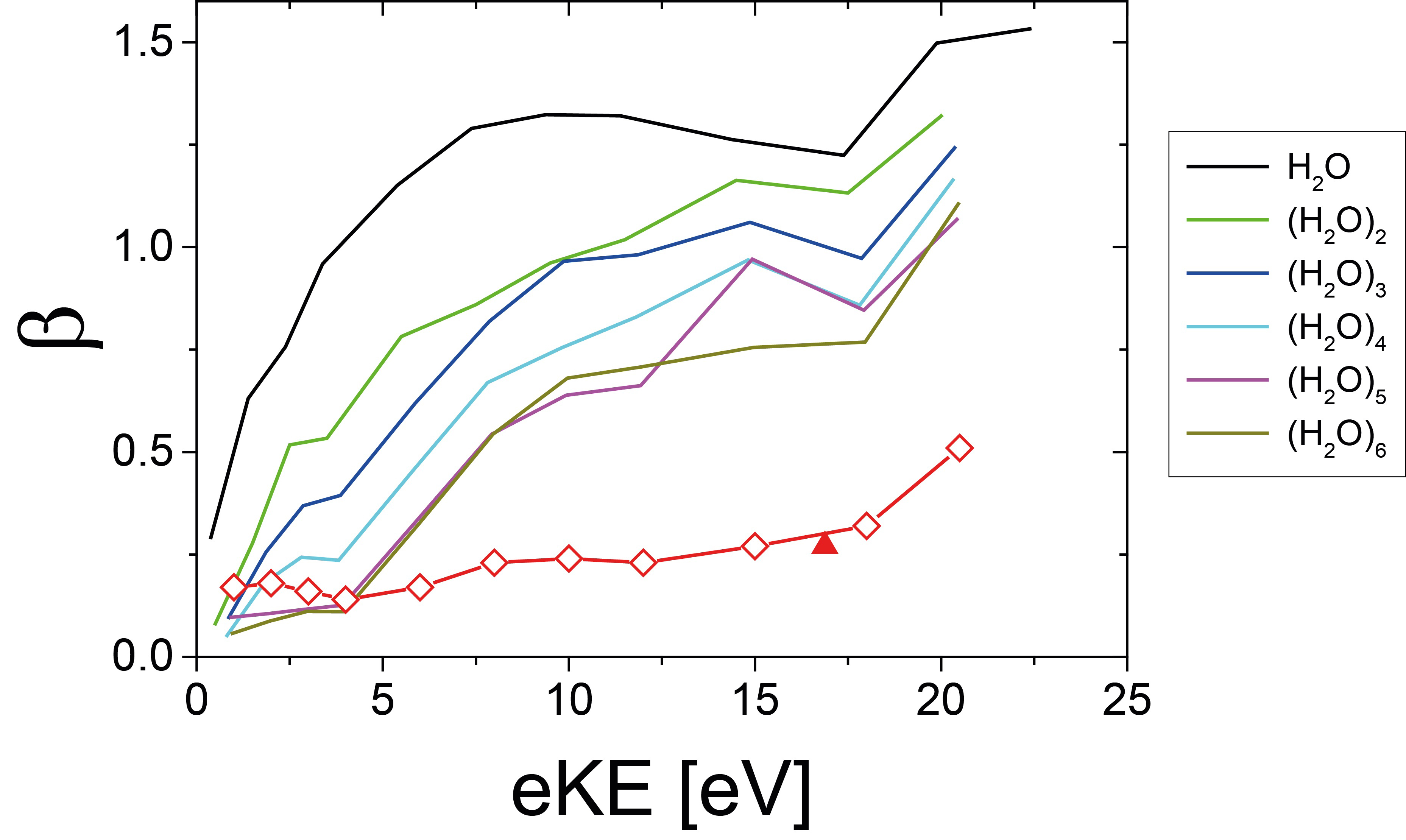
This issue was addressed by the ETH/SOLEIL scientists who recorded angle-resolved photoelectron spectra of water clusters of different size containing up to 20 individual water molecules. These clusters are so small that any contribution from the second scattering process (Fig. 1b) can be neglected and thus allows the investigation of the first process only (Fig. 1a). The double imaging photoelectron/photoion coincidence (i2PEPICO) spectrometer of the molecular beam chamber SAPHIRS available on the DESIRS beamline was crucial for these studies because it provides cluster-size specific information and avoids issues with the dominant overlapping water monomer contribution. Information on the scattering features of the photoionization process itself was obtained through the analysis of the kinetic energy and the angular distributions of the acquired and mass-filtered photoelectron images. The evolution, with the cluster size, of the photoelectron angular distributions (PADs) asymmetry parameter (b) as plotted in Fig. 2 revealed that the contribution of the photoionization process (Fig. 1a) is fully described by a water cluster with approximately six water molecules. This size corresponds to the first solvation shell and to the smallest cluster size for which ring-topology structures become less stable than three-dimensional hydrogen-bond networks.
Electron scattering in liquid water
The experimental cluster data and the two step model depicted in Fig. 1 allowed the researchers to predict electron scattering in liquid water (red diamonds in Fig. 2). The usefulness of this approach was recently confirmed by the first experimental liquid water data by Nishitani et al., Struct. Dyn., 4, 044014 (2017) (red triangle in Fig. 2).
This work opens the way for a better understanding of slow electron scattering processes in liquid water induced by radiation.