DESIRS beamline - Ring-Opening Dynamics of the Cyclopropyl Radical and Cation
Rings are one of the primary ways molecules build three-dimensional structures. Their properties and dynamics are therefore of central importance across many chemical fields. Characterizing the triangular geometry and associated intense inherent strain of cyclopropyl (C3H5), one of the smallest, simplest, and most fundamental cyclic molecular motifs, has accordingly intrigued chemists for over a century. Answers to questions about the stability of such rings in radical or cationic forms have implications for physical understanding of molecular behavior and applications in modern organic synthesis.
The study set out to answer unresolved questions regarding the kinetic and thermodynamic stability of the cyclopropyl radical* and cation. Due to the reactivity and instability of the cyclopropyl radical, only a few prior experimental studies existed, out of which only one photoelectron spectroscopy study bore direct relevance to the thermodynamic and kinetic questions at issue here. The combination of several capabilities at SOLEIL was ideal for attacking this problem with modern methods: specifically an F atom abstraction reactor for in situ radical generation, synchrotron vacuum ultraviolet (VUV) radiation at the DESIRS beamline and the DELICIOUS3 double imaging photoion-photoelectron coincidence (i2-PEPICO) spectrometer.
After being produced in the reactor, the C3H5. Radicals—along with other by-products—are then irradiated by the VUV produced on the DESIRS beamline, leading to photoionisation: C3H5. -> C3H5+ + electron.
The different chemical species present are then analysed using the i2-PEPICO spectrometer which associates the corresponding photoelectron spectrum to each species seen in the mass spectrum.
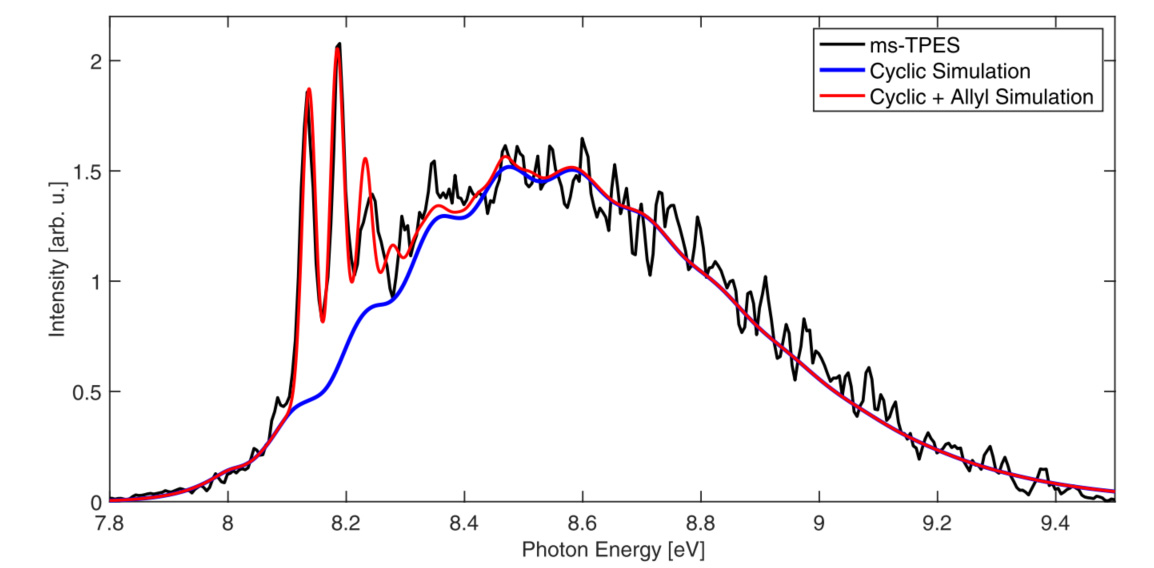
In addition, using innovative calculations based on the propagation of vibrational wave packets in the potential energy surfaces of the cation, the scientists modelled the theoretical photoelectron spectrum expected for the cyclopropyl cation, but also its linear form (allyl) which is produced by ring opening (figure 2).
These experimental and theoretical results were obtained by a collaborative effort between scientists at Ben-Gurion University of the Negev, Harvard-Smithsonian Center for Astrophysics, Institut des Sciences Moléculaires d’Orsay, Université Bordeaux, University of Florida – Gainesville, Argonne National Laboratory, and at the DESIRS beamline.
The experiment/theory comparison provided refined values for the dissociation energy of the C-H bond in cyclopropane and the cyclisation energies of cyclopropyl radicals and cations, among other important thermochemical quantities.
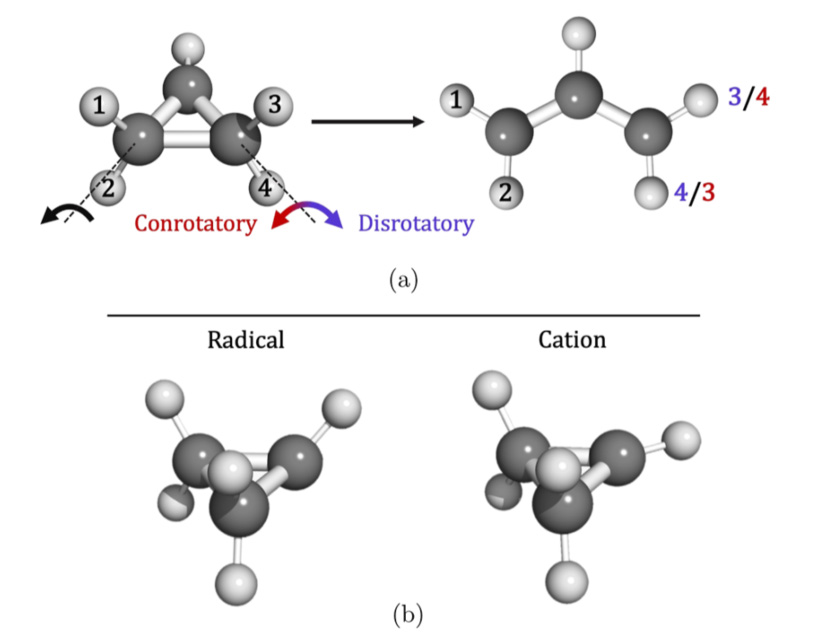
The close agreement between experiment and theory allows for the convincing conclusion that the cyclopropyl cation does not, in fact, exist as a stable species, but is rather a transition state between allyl cation structures. The cyclopropyl radical then exists because of kinetic barriers to ring-opening, which are not present in the disrotatory opening of the cation (figure 3). This settles a basic question going back to the early theory of pericyclic reactions (in which a molecule goes through a transition state with a cyclic geometry).
* Free radical (or radical): a chemical species with one or more unpaired valence electrons on its outer electron shell - each unpaired electron is noted by a dot. The presence of this electron (or electrons) often makes the radical highly unstable, with a very short lifetime, and it can react with many compounds in mostly non-specific processes.