Crimean-Congo Hemorrhagic Virus - Structural biology results for future vaccines and treatments
Crimean-Congo hemorrhagic fever (CCHF) takes its name from a first description of this disease in Crimea in 1944, among Soviet soldiers, and then in Congo in 1956. Transmitted to humans by a tick bite, or by direct contact with bodily fluids of an infected animal or person, it is fatal in 10 to 40% of cases.
An international team of researchers has provided new information on the structure of CCHF virus antibodies and the mechanism of protection against this virus. Their results are based on experiments conducted at three synchrotrons, APS (USA), ESRF (Grenoble) and on the PROXIMA-2A beamline at SOLEIL. The results are published in the journal Science.
Infection by the CCHF virus first causes fever, headache, muscle pain... Then, the illness progresses into a hemorrhagic phase, which is fatal in 10 to 40% of cases. The WHO currently has a list of 10 diseases prioritized for research and development within emergency contexts. On this list is the CCHF virus, which is endemic in Eastern Europe, Asia, Africa and the Middle East. However, with global warming impacting on animal habitats and migratory patterns, this virus could infiltrate new regions where it previously didn’t exist. Recent cases in Spain, Italy and the UK are thought to have originated from ticks transported by birds. Thus, CCHF poses a substantial threat in transitioning from a regional endemic to the next global pandemic.
Very little is known about the structure of CCHFV-directed antibodies, and the mechanism that the body uses to neutralize the virus. This presents a significant barrier to the development of life-saving medical treatments and vaccines for communities where CCHF virus is endemic.
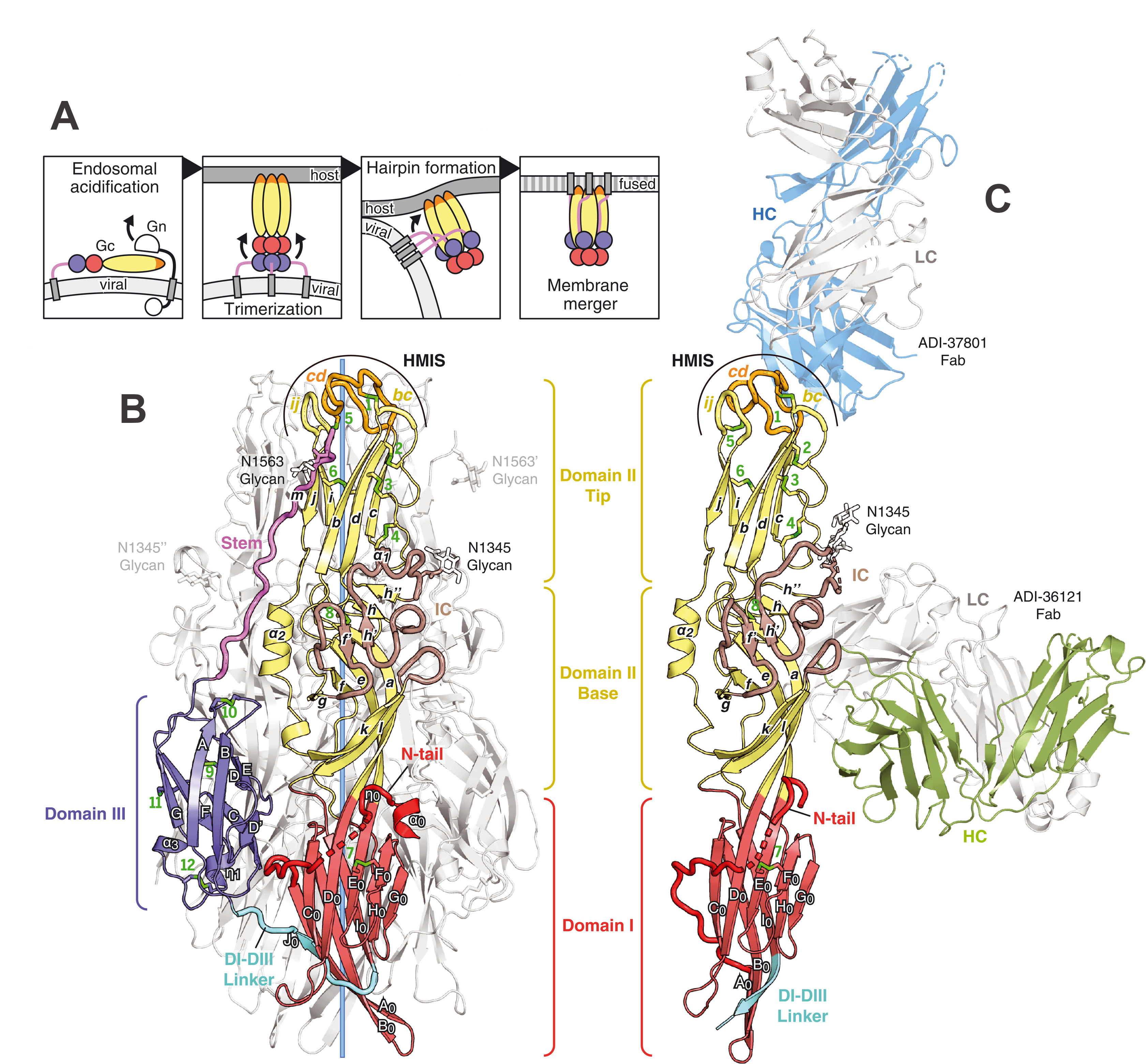
Specifically, it is known that the CCHF virus infects host cells through glycoproteins Gc and Gn, which undergo conformational changes upon fusion to the host cell membrane. Yet it is not known how the most potent CCHFV antibodies, ADI-36121 and ADI-37801, neutralize the virus. Using X-ray diffraction data, the team of scientists was able to solve the 3D structures of post-fusion Gc and pre-fusion Gc bound to the antigen-binding fragments of ADI-36121 and ADI-37801 ; see Figure 1 : The 3D structure of the trimeric post-fusion form of the protein (B) was obtained using X-ray diffraction measurements performed at the ESRF (ID23-1 beamline) and SOLEIL (PROXIMA-2A beamline) ; the structure of the antibody complex (C) was obtained using X-ray diffraction measurements performed at the APS (19-ID-D beamline).
Uniquely, their results clearly show the two antibodies working synergistically to prevent Gc-driven membrane fusion by blocking glycoprotein conformational changes.
Although more insights are needed to develop therapeutics and vaccines for limiting and preventing the spread of CCHFV, this research provides fundamental insights for the basic reactivity needed for vaccine discovery and development. Due to the fact that CCHFV is already endemic around the world and has a significant impact on human life, we should be directing resources towards the development of vaccines for this disease today. In fact, some pharmaceutical companies, like Moderna, are currently exploring CCHFV vaccines for clinical trials.