Condensation effects on electron chiral asymmetries in the photoionization of Serine: from free molecules to nanoparticles
Researchers from the DESIRS beamline have determined for the first time chiral* asymmetries (caused by photoelectron circular dichroism (PECD*)) in photoelectron imaging experiments on chiral aerosol particles. Such asymmetries, observed as a preference for photoemission along or against the direction of light propagation, can in principle be used to obtain information on local order, molecular conformations or supramolecular chirality. However, such asymmetries are difficult to measure for aerosol particles, because as compared to the gas phase, they are blurred by electron scattering processes and superimposed onto dominating particle specific non-chiral asymmetries caused by the so-called shadowing effect.
This proof of principle study was conducted on the chiral amino acid serine and compares data obtained for serine aerosol particles with data obtained for different conformers* of free serine molecules brought in the gas phase by aerosol thermodesorption.
Words marked with an asterisk are explained in the glossary below
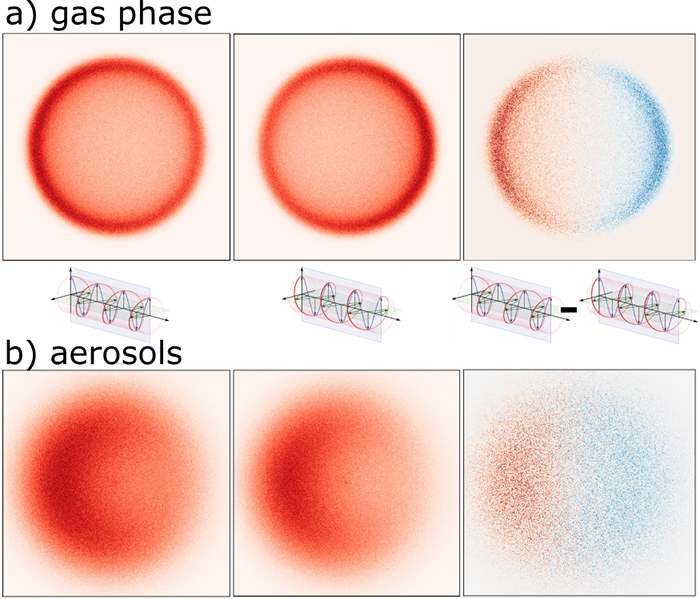
The comparison of photoelectron velocity map imaging (VMI) data between gas phase and condensed species (e.g. aerosol particles) shows a series of pronounced differences. Upon condensation, the ionization energies* shift to lower values and photoelectron bands are broadened, marking the emergence of an electronic band structure. The photoelectron angular distributions are affected by condensation effects as well: In addition to changes of the electronic orbital character and molecular conformation, which affect the angular distribution* of photoemission (i.e. the usual β parameter* or the PECD), photoelectron scattering within the aerosol particles tend to make the distribution more isotropic. Furthermore, the so-called shadowing effect creates a particle specific forward/backward asymmetry in the VMI images that can be explained by considering that the half of a spherical particle facing the light source tends to emit more electrons than the half facing away. This shadowing effect is often much more pronounced than the usual angular anisotropy (β parameter or PECD) thus dominate the visible asymmetries of aerosol photoelectron images.
The differences between gas phase and aerosol VMI images are illustrated by the simulated images in figure 1.
For gas phase molecules, double imaging photoelectron photoion* coincidence spectroscopy (i2PEPICO), as implemented on the DESIRS beamline, enables to record photoelectron images selected by the mass of the photoion (parents or fragments) formed after photoionization. In the specific case of serine the fragmentation of the photoions depends on the molecular conformation, so that photoelectron spectra and PECD for individual molecular conformers* can be recorded. Figure 2 shows this data for the three most abundant conformers recorded at a photon energy of 12.4 eV. The PECD in the bottom panel gives the magnitude of the difference image (see figure 1) as a percentage of the signal intensity in the individual images. It is clearly observable that not only the photoelectron spectra of the three conformers differ (in ionization energy and relative signal levels for different bands), but also the magnitude (of the order of a few %) and energy dependance of the PECD, which is sensitive to the molecular potential by which the outgoing photoelectrons are scattered off.
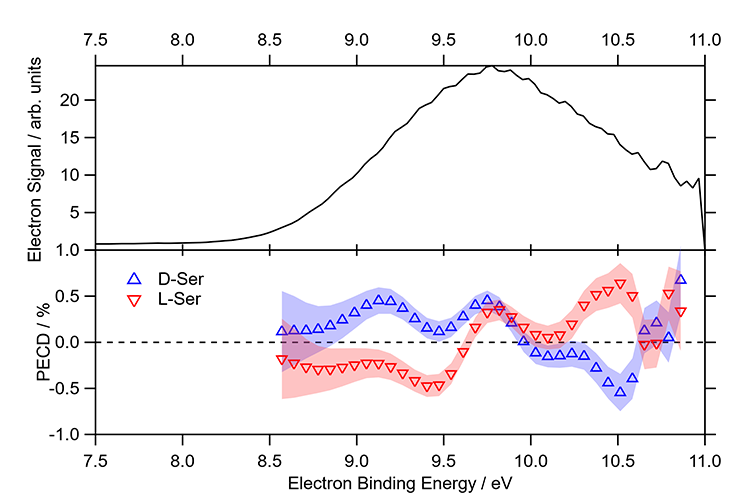
The photoelectron spectrum of serine aerosol particles recorded at 11 eV photon energy is displayed in figure 3. Although the PES does not show any resolved structure, the PECD, shown by the mirrored red and blue traces displaying data obtained for D- and L-serine homochiral aerosols, exhibit a clear dependence on the electron binding energy. The PECD for aerosol particles is reduced in magnitude as compared to the one obtained for gas phase conformers but remains clearly detectable (of the order of ~0.5 %), with clearly observable qualitative differences with respect to the data obtained for gas phase conformers. This survival of PECD-related asymmetry is quite surprising since aerosol photoelectron images are often assumed to be isotropic because of strong electron scattering processes that blur out the angular dependencies. The non-vanishing PECD-induced asymmetries in chiral aerosols might be due to a local order effect or to fewer conformers used to build up the aerosols, both effects which would increase the chiral asymmetry, somehow partly compensating the scrambling effect and loss of initial polarization memory due to scattering processes.
This study shows that it is possible to determine chiral asymmetries (PECD) and possibly also photoelectron anisotropy parameters (β-parameters) for aerosol particles. Especially PECD offers a promising avenue for future studies of condensation or solvation effects on molecular conformation and electronic structure of biologically-relevant molecules/nanoparticles.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------
GLOSSARY
Chirality / enantiomer: An object is “chiral” if it exists as two non-superimposable forms which mirror image one of the other. This is the case of hands (or feet!), propellers, but also of many molecules (for example, the ones with an asymmetric carbon linked to 4 different chemical groups) – the two forms are called left or right enantiomer.
Amino acids / homochirality: the 22 amino acids, building blocks of proteins, are all chiral except the simplest one, glycine. in the biosphere, one only finds the left-hand form (enantiomer) of chiral amino acids (and the right-hand form of sugars in DNA and RNA). This is homochirality of life.
Conformers: like many other molecules and in particular biomolecules, amino acids are floppy molecules which exist as several conformers, obtained by rotations of chemical groups around a chemical bond, for example of the carboxylic group (COOH) or the amino group (NH2) around the bond that binds them to the central asymmetric carbon.
Circularly Polarized Light (CPL): Polarization is a geometric property of light, which describes the motion of the electric field associated with the light wave as it propagates. In the case of circularly polarized light, the electric field describes a helix (like a corkscrew) which can be clockwise or counterclockwise, defining then CPL with left or right helicity. Note that CPL is a chiral object.
Photoemission / photoionization: action of light tearing off an electron from matter. It corresponds to the photoelectric effect: the energy of the incident photon (hν) is shared between extraction work of the electron (binding energy) and the kinetic energy of the emitted photoelectron (Ek). The compound (atom or molecule) from which light has torn an electron is “photoionized” / becomes a photoion.
Photoelectron spectroscopy (PES) measures the distribution of Ek energies for a given incident photon, allowing the determination of the (binding) energy levels of the electrons in the molecule (which corresponds to the molecular orbitals energies) . One can also measure in which directions the photoelectrons are ejected with respect to a given axis: i.e angular distributions. This distribution (characterized by the β parameter) does not have the same properties in all directions, in other words: is not isotropic (β ≠ 0) because the molecule "remembers" the polarization of the incident light.
PhotoElectron Circular Dichroism (PECD): As LPC is a chiral object, it will induce enantio-specific processes upon interaction with a given enantiomer of a chiral species: these are circular dichroisms, a chiral recognition between chiral objects, such as a left glove "recognizes" a left hand. In the case of photoemission of a given enantiomer of a chiral molecule by an LPC of a given helicity (left or right), the PECD manifests itself by a forward / backward asymmetry with respect to the light propagation axis, which is not found for non-chiral species. This asymmetry is reversed when the enantiomer or light helicity is swapped. The specificity of PECD, as compared to other types of circular dichroisms, lies in its intensity: from a few % to a few tens of %. It is also very sensitive to molecular structures such as isomers (molecules with the same atomic composition, but a different arrangement of these atoms), or conformers.