Chirality reveals electron/nuclear motion couplings
The coupling of electronic and nuclear motions is a striking manifestation of the complex many-particle dynamics involved in off-equilibrium systems, like excited molecules.
The coupling of electronic and nuclear motions is a striking manifestation of the complex many-particle dynamics involved in off-equilibrium systems, like excited molecules. Such a coupling may be revealed, in the case of molecular electronic transitions, by the breakdown of the so-called Franck-Condon (FC) approximation. This approximation is widely accepted, as is its assumption of vertical transitions under the Born-Oppenheimer approximation (separation of the nuclear and electronic motions in view of their very different masses). A secondary FC-assumption is that the probability of a given electronic transition does not depend on the nuclear geometry sampled during vibrational motion. Consequently, there can be an expectation of fully decoupled electron-nuclear dynamics. In the case of molecular photoionization, this means that the electron continuum features should be decoupled from the vibrational energy content of the resulting ion. Departures from this picture are of course encountered, most usually attributed to shape resonances in the continuum.
In this context, chiral molecule systems can offer an enhanced opportunity for investigating molecular photoionization, including vibrational dynamics, in great detail, even for randomly oriented samples. Indeed, for such chiral systems the laboratory-frame angular distribution of photoelectrons produced by circularly-polarized light (CPL)-induced photoionization, can present a strong forward-backward asymmetry with respect to the light axis, called Photoelectron Circular Dichroism (PECD). This effect can survive averaging over random molecular orientation, because CPL defines a direction in space, with respect to which the different atoms of a given molecular enantiomer will be seen always in the same configuration by the incoming photon.
Quantitatively, the PECD asymmetry equals 2b1, where b1, the so-called dichroic parameter, which encapsulates the chiral contribution is anti-symmetric with the swapping of the either the enantiomer or the light handedness. It has been shown that PECD measurements can be used to probe molecular geometry and (static) structures, including conformers and chemical substitution. PECD appears therefore as an ideal tool to study also the dynamic interplay between electronic and nuclear motions upon photoionization.
The case of methyloxirane (fig.2), photoionized just above its ionization energy, a region far from any shape resonance, with the CPL VUV light of the DESIRS beamline, was investigated. The corresponding electrons are then imaged onto a position sensitive detector via a Velocity Map Imaging spectrometer that provides in a multiplex way Angle-Resolved Photoelectron Spectroscopy (AR-PES), with an 100 % collection efficiency and an ultimate 5 % kinetic energy resolution.
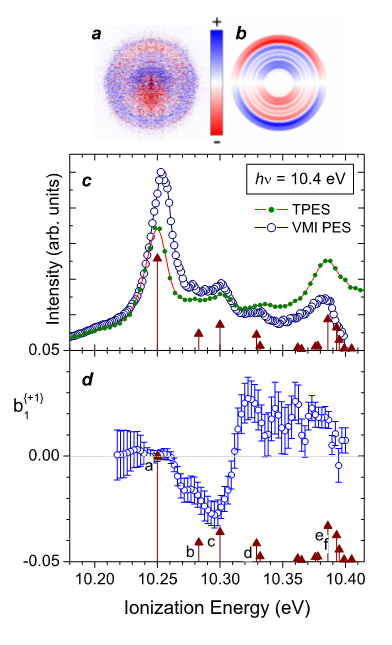
From the radial distribution in the total electron image we obtain the PES, while from the leftCPL-rightCPL difference images the dichroic parameter, b1 , can be obtained. The difference images (fig.1a-b) readily reveal a marked vibrational structure in the radial (i.e. electron energy) distribution, associated with strong forward-backward asymmetries in the angular distribution. Dramatic changes in the sign of the latter for some adjacent vibrational ring patterns can also be seen in the alternating color mapping.
The vibrational structure identified in the PES (fig.1c) - the origin band (peak “a”) and a series of single quantum excitation of skeletal deformations (peak “b” to “f”) - exhibit very distinctive and remarkable dichroic behavior, with b1 values (panel d) ranging from a null value for peak “a”, to a negative value of -0.03 for “c”, and then switching sign within a few tens of meV to a positive ~ +0.02 value for peaks “d” and “e”. Phenomenologically, this sign change in b1 means that the forward/backward asymmetry reverses direction for adjacent individually resolved vibrational modes. This striking behaviour of asymmetry flipping, upon vibrational (adjacent) excitation has never been observed, to our knowledge, in any molecular photoionization experiment. Moreover, this dependence of PECD with the vibrational energy content of the residual cation is completely unexpected within the usual FC assumptions.
This dramatic vibrational effect is probably due the known sensitivity of PECD to static geometric structure, which would be somehow replicated in dynamic structural changes occasioned by the vibrational motion sampling different regions of the nuclear configuration space.
This photoionization vibrational effect in PECD appears more remarkable than vibrational effects measured in the past on fixed-in-space molecules, yet it has been achieved without requiring molecular orientation because of the intrinsically chiral nature of the targets. In this context, PECD appears to offer a powerful, and (for chiral species) universally applicable probe of vibrational dynamics in molecular photoionization, even from randomly oriented targets. This, of course, is the “natural” situation for the ubiquitous chiral molecules in the biosphere.
Besides its fundamental interest, this finding may have important consequences for the interpretation of PECD in an analytical context, with the quickly-growing field of laser-based multi-photon PECD experiments whose outcomes may be driven by vibrational dynamics in both the intermediate and final state.