Chiral clusters: a molecular handshake probed by photoelectrons
Through a long standing collaboration between the University of Nottingham and the DESIRS beamline team, scientists have used the technique of photoelectron circular dichroism (PECD) to study chiral recognition on the glycidol “prototype” molecule.
Molecular docking at a suitable receptor site, enabled by the “lock and key” principle, has been central to understanding of biomolecular activity for many years. At its simplest this requires a matching of molecular shapes at the receptor site to guarantee a successful fit of the interacting ligand species. This concept acquires particular significance when the reagent is chiral as, rather like trying to insert a left hand into a right handed glove, the docking becomes chirally selective. Many biomolecular interactions are in fact chirally specific owing to homochirality (i.e. the overwhelming dominance of e.g. L-amino acids in the terrestrial biosphere). Consequently, many chiral pharmaceuticals are enantioselective in their action, many chiral pairs of natural products are perceived to have distinct odours, and in the insect world pheromones operate with chiral selectivity. Ideas of a matching shape need, however, to be refined to take account of the actual molecular interactions that take place. Chiral molecular recognition aims to understand the matching of shape by reference to the weak interactions, such as hydrogen bond formation, that occur in a successful docking process.
Chiral self-recognition in the formation of small H-bonded clusters of the molecule glycidol (C3H6O2 – see figures) provides a valuable prototype for chiral recognition studies. But although mass spectrometry seemingly provides a direct way in which to identify the size of gaseous clusters, there is a problem that, owing to the relatively weak bonding mechanism, they may fragment upon ionization, giving misleading indications of the nascent cluster sizes – although once identified this is in itself a useful source of information about the strength of cluster binding.
There is therefore a need for a direct chiroptical probe of chiral clusters. Such an opportunity is provided by the chirally discriminating technique of photoelectron circular dichroism (PECD), which detects an enantio-specific forward-backward asymmetry in the spatial distribution of electrons emitted upon ionization by a beam of circularly polarized radiation. This asymmetry is detected as positive/negative regions in a photoelectron image formed from the difference the 3D electron distributions observed with alternating left and right circularly polarized light. Fig 1. PECD is known to be a long range sensitive probe of whole molecular structures which therefore should unravel structural information regarding the clustering process.
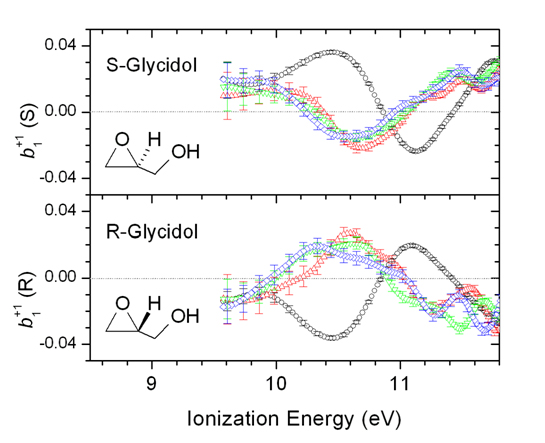
Through a long standing collaboration between the University of Nottingham and the DESIRS team, scientists have used the DELICIOUS 2 electron/ion spectrometer, to record electron images in coincidence with specific mass-selected cluster ions, with sizes n=1,4. The PECD experiment quantifies the chiral asymmetry in a spectrum of a single energy dependent parameter, b1. The PECD spectra of the monomer and dimer ion show dramatic differences (Fig. 2), indicating the different neutral precursor species; the higher n-mers have rather more similar spectra, suggesting that some observed dimer evidently results from cascading fragmentation of the higher n-mers. Supporting evidence for this inference was obtained from a measurement made below the monomer adiabatic ionization energy. At photon energies this low the still observed monomer ion cannot be directly produced, but only results as a fragment of larger clusters (which have lower ionization thresholds). Crucially now, the PECD of “monomer” is indistinguishable from that of the dimer and trimer, indicating a now common neutral parentage for these cluster ions.
Beyond this striking phenomenological m/z-dependence of the PECD signal, the next step consists now in using the newly-commissioned DELICIOUS3 double imaging spectrometer, allowing to filter electron images not only for a given m/z but also for a given kinetic energy release. This should allow the disentangling of cascading dissociation processes providing a genuine size-selection of the various nascent clusters. In the particular case of the Glycidol dimer, the resulting filtered PECD value should give access to structural information on the homochiral dimer configuration (the molecular handshake), by comparison with PECD theoretical calculations.