The Cabrera-Mott model of metal oxidation confirmed more than 70 years after its publication, at TEMPO beamline
In 1949, the Nobel laureate in Physics sir Nevill Mott together with Nicolás Cabrera laid the foundations of the theory of the oxidation of metals. Today, the application of this model is still widespread, even in nanofabrication.
Researchers from LCPMR (Sorbonne Université) in collaboration with TEMPO beamline present a methodology based on ambient-pressure X-ray photoemission spectroscopy (AP-XPS) to study thin oxide film growth in real time and test the implied tenets in the model in the case of aluminum. It can further prompt the analysis of other technologically relevant metals, for example, the ones used in superconducting qubits for quantum computing.
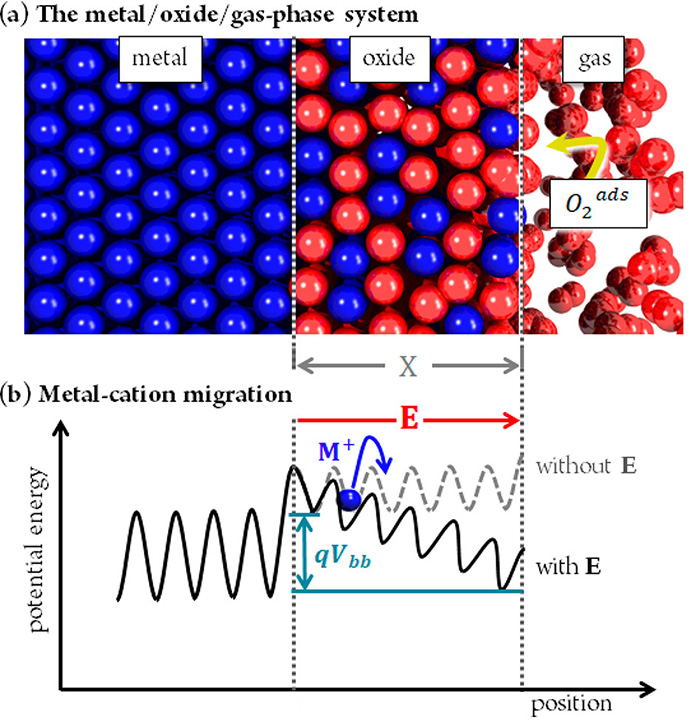
Oxidation phenomena on metal surfaces play a key role in many technological fields, ranging from corrosion science to electronic device design. Thence, they cover a vast field in basic scientific research and have attracted considerable attention from both the experimental (Transmission Electronic Microscopy, XPS, surface potential measurements) and the theoretical (Density Functional Theory calculations) points of view. Most of these studies have been interpreted using the Cabrera-Mott (CM) theory, which is based on a model that assumes thermodynamic equilibrium for the electrons, from the metal to the oxygen adsorbate-covered oxide surface. The core idea of this theory establishes that the transport of ions during the oxide growth is assisted by a built-in electric field E deriving from the so-called CM potential (see Figure 1). This electric field stems from the negative charging of the adsorbed oxygen anions and the opposite positive charge that appears on the metal side (as in a parallel plate capacitor).
In this 70-year timespan the CM model was essentially tested via oxide growth kinetic studies, which did not provide a thorough description of the metal/oxide/gas interface. The direct proof that the CM potential (and the field E) is established in the growing film had to wait for the advent of AP-XPS. Indeed, this is the only tool capable of giving a detailed picture of the electronic structure at the gas/solid interface.
By combining an original thermodynamic approach with precise measurements of the XPS binding energies, the authors were able to verify the tenets of the CM model in the case of aluminum oxidation and interpret changes in band structure and oxidation rate as a function of O2 pressure (up to 1 mbar) with the famous CM rate law.
Besides aluminum, AP-XPS will be profitably used to study the oxidation (or corrosion) of many metals of technological importance. Moreover, its utility can be certainly extended to the analogous case of gas sensors in working conditions.