Biology-Health: Mechanism of biosynthesis of iron-sulfur clusters in cells
Iron is an essential element for life. It is used in the form of iron-sulfur clusters as a constituent of active sites of enzymes involved in vital processes such as the storage of energy in ATP (adenosine triphosphate), the synthesis of cellular constituents and the maintenance of genome integrity.
The biosynthesis of iron-sulfur clusters is a complex process that is still poorly understood. Moreover, a number of diseases are caused by a defect in this process. It is therefore important to better understand the mechanism of assembly of these clusters. The study presented here determined the first structure of the iron site in the scaffold protein that ensures the assembly of these clusters.
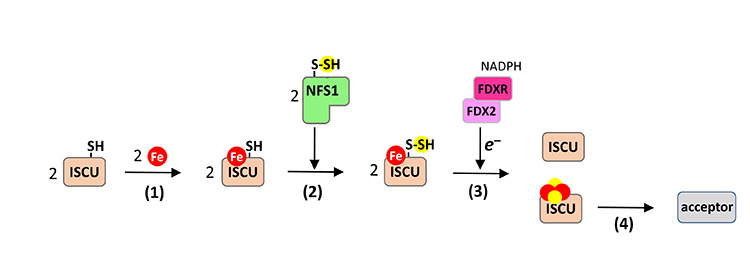
Iron-sulfur (Fe-S) clusters are polynuclear assemblies of iron and sulfide ions that constitute the active sites of a very large number of enzymes involved in a multitude of essential biological processes. Fe-S clusters are biosynthesized into [2Fe2S] and [4Fe4S] forms by specialized multi-protein machineries and are then inserted into target proteins. The biosynthetic process is carried out in several steps, most of which are still poorly understood (Fig. 1).
In a previous study, scientists from I2BC, ICSN and the University of Kaiserslautern involved in the study described here showed that, in the mouse ISCU protein - the scaffold protein for Fe-S clusters - iron binding precedes sulfur insertion (Fig. 1, step 1).
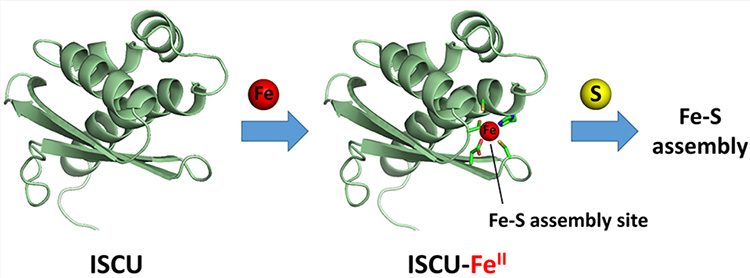
Their measurements by Mössbauer and electron absorption spectroscopies had shown that iron binds in the ferrous form (Fe2+) via several sulfur atoms from cysteine amino acids in the ICSU protein. The assembly site of the Fe-S cluster in ISCU contains three cysteines as well as a histidine and an aspartate, which are strictly conserved amino acids between species. The mouse ISCU protein also has a fourth cysteine but this does not belong to the assembly site and is not conserved between species. Furthermore, their studies by site-directed mutagenesis had led to the conclusion that at least three amino acids of the ICSU assembly site are involved in iron binding: the aspartate and two of the cysteines (n°35 and 61). The researchers therefore hypothesized that iron binds in the assembly site, but the complete structure of this site had yet to be determined.
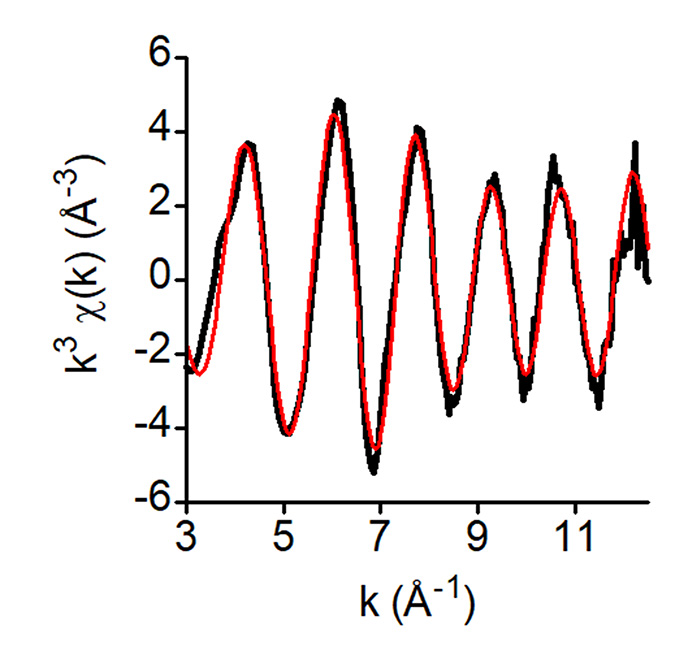
Their X-ray absorption spectroscopy measurements on the SAMBA beamline showed that iron binds to ISCU in a cysteine-containing site and they determined the first structure of the iron center in the mouse ISCU protein. According to the analysis of the EXAFS data, iron is in a mononuclear form bound by four atoms: two sulfurs and two nitrogens and/or oxygens (Fig. 2).
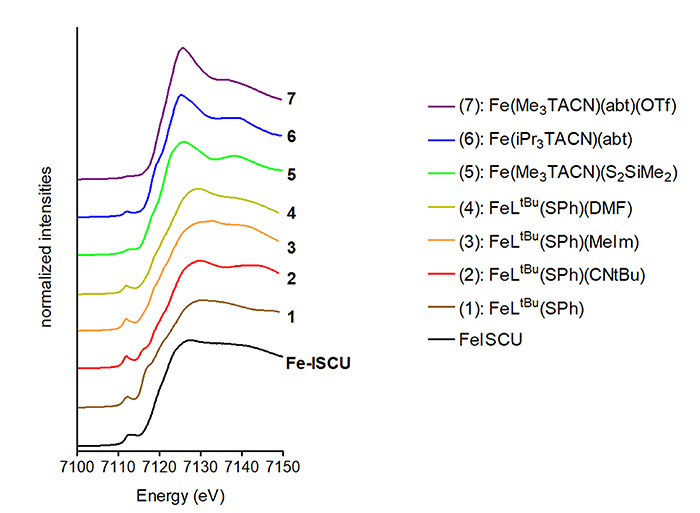
Analysis of the XANES data and comparison with model iron complexes showed that the iron is in a tetrahedral environment bound by four ligands, in agreement with the EXAFS data (Fig. 3).
These data allowed the researchers to propose a structure for the iron center in the assembly site in which the iron ion is bound by 2 cysteines (Cys35 and Cys61), an aspartate (Asp 37) and a histidine (His103), arranged in a tetrahedral geometry.
From all these results, it appears that iron binds in the assembly site to initiate the assembly of Fe-S clusters. The determination of the structure of the iron site by X-ray absorption spectroscopy now opens up the possibility of studying in greater details the mechanism of sulfur insertion and ultimately the formation of Fe-S clusters.