Are surface stress and surface energy of nanoparticles size-dependent?
In Materials Science, how to simultaneously analyze the morphology and the properties of nanoparticles? This case has motivated a collaboration between LUCIA beamline and Paris Institute of Nanosciences (CNRS - UPMC-Sorbonne Universités). Their results are published in Nano Letters.
Surface energy and surface stress are the reversible works required to create a surface unit area and to elastically stretch a pre-existing surface unit area, respectively. They are central in the thermodynamics description of materials. This is particularly true at the nanoscale where the increase in surface-to-bulk ratio results in size-dependent behaviours with equations in the form: surface energy/size or stress/size. For example, the decrease with size of the melting temperature of small particles (Gibbs-Thompson equation) explains why ice cream gets crunchier over time. However, an utterly controversial issue is the size dependence of surface energy and stress. For metal nanoparticles, including gold, copper, silver and palladium, estimates often span over an order of magnitude, far away from bulk values, and, what is even more confusing is that no physical reason is proposed to explain those discrepancies.
Such blurred landscape points to the lack of relevant methods to simultaneously analyze the morphology and the properties of nanoparticles, a long-standing problem in materials science. The case has motivated a collaboration between LUCIA beamline and Paris Institute of Nanosciences. In situ X-ray absorption and UV-visible reflectance are combined to atomistic simulation to determine the size, shape, structure as well as the adhesion energy of growing Ag/α-Al2O3(0001) nanoparticles.
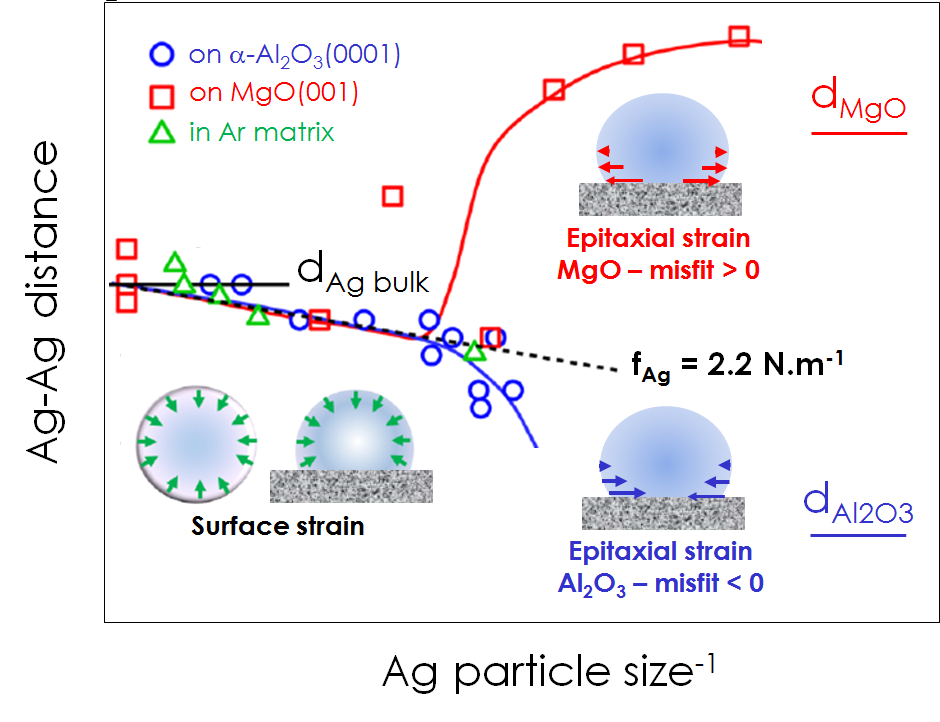
The comparison of the three sets of EXAFS data shown in figure - the present Ag/α-Al2O3(0001) (negative misfit -5 %), previous Ag/MgO(100) (positive misfit 3%) and Ar-embedded silver - demonstrates a unique size-dependence for silver nanoparticles above 3 nm in size, no matter whether they are unsupported or supported on one or the other substrate. Changes in the Ag-Ag distance that accompany changes in size of the silver clusters feature a straight line whose slope gives the surface stress fAg = 2.2 N·m−1. In contrast, for silver clusters < 3 nm in size, values of the Ag-Ag distance tend to compensate misfits (Figure).
Thanks to the combination of x-ray absorption, UV-visible reflectance and numerical simulations that was shown of great relevance to characterize growing nanoparticles, the question raised initially received a first response. The robust size-independent value of the surface stress of silver (fAg = 2.2 N·m−1) that has been determined is based on sound physics and valid for all objects except extremely small particles (< 3 nm). Since the observation mostly relies on surface/bulk ratio, a metal-independent picture emerges that is expected to have far-reaching consequences for the understanding of the controversial energetics of nanoparticles.